The 2025 Longevity Summit Dublin was held in July, and we have the highlights from the event for you along with the latest research updates and news from the conference.
The Summit was founded by Aubrey de Grey and Martin O’Dea in 2022 and combines a plethora of noteworthy researchers and advocates in the rejuvenation biotechnology field.
Hosted in Dublin at the historic Trinity College, it is Ireland’s leading university, ranked No. 1 in Ireland and 87th best in the world. It was founded in 1592 and has a rich history and a reputation for excellence in education, research and innovation. Famous writers Oscar Wilde and Samuel Beckett, and scientists William Rowan Hamilton and Ernest Walton, attended Trinity College.
This makes it the ideal place to host a conference focused on the frontiers of biology, technology and human innovation. Since its inception in 2022, the Summit has cemented its place in the longevity event landscape.
Day one: Longevity goes public
The event was a four-day affair, with the first day free and open to the public and devoted to women’s health and aging. This area of research is neglected, in particular that of female reproductive aging and female aging in general. It was good to see that this was the theme of the first day.
Having the first day of the conference open to the public is a clever move. Like it or not, aging and rejuvenation research is a niche topic, and it’s not one that the public is likely going to pay to learn about. So, having a day free to attend means that curious people can whet their appetites, and it helps to spread the word.
While we are some years away from the first therapies reaching the healthcare system, there are quite a few rejuvenation therapies in clinical trials right now. Mass public awareness and support is unlikely until these therapies begin to arrive. At that moment, the acceptance that aging decline is something we can address is likely to become widespread.
Day two: Longevity talks begin
The second day saw the start of scientific talks that were targeted at researchers and people who are invested in the field. There was a mix of speakers this year, and some of the talks were particularly impactful.
Healthspan and lifespan are mutual goals
Martin O’Dea welcomed guests with his opening talk. Martin is a well-known figure in the longevity community, an experienced businessman, an author, and the CEO of Longevity Events Limited, which hosted the Longevity Summit Dublin.

He reflected on the recent trend of longevity and how the conference was trying to combine public interest in healthspan and well-being with the actual science of rejuvenation. He also cautioned that while he believed the two things could work together, it was important to ensure that science remained the focus for the event.
Martin suggested that lifespan and healthspan aren’t in opposition but that they need to be combined to work together and not in opposition. He said, “We can chew gum and walk; we can do healthspan and lifespan together.”
The idea that lifespan and healthspan are separate and competing goals makes little sense when you think about the actual biology at play here. It is not impossible to increase one but not the other, the modern healthcare system has achieved this by increasing lifespans but not healthspans, but the two are strongly linked.
The goal of our field is not to increase one over the other. The aim is to provide both longer and healthier lifespans for everyone. Ultimately, this is about quality and quantity, and this is what Martin was driving at in his opening talk.
Nanotics could really shake up rejuvenation biotechnology
The first presentation was by Lou Hawthorne from biotech company NaNotics, which pushes the envelope in trying new things. Its approach is to use NaNots to remove target molecules from blood without the use of drugs or external filtering devices.

NaNots are tiny artificial structures that lock in the target molecules away, making them ready to be excreted by the body. One advantage of NaNots is that they can remain in blood for days while absorbing these molecules.
This company’s current focus is on tumor necrosis factor (TNF), a regulator of the immune system and inflammatory response. NaNots only target the soluble TNF in blood and ignore membrane TNF, which is important as the membrane-bound form of TNF is very important for healthy cell function. NaNots are able to be selective in this manner, unlike traditional drugs that can target both forms.
Lou mentioned that the soluble form of TNF is linked to multiple sclerosis (MS) and that the company is hoping to move to the clinic to try to cure it. He reported that the mouse data they have for the approach is promising enough to move towards a clinical trial.
NaNots may also potentially be applied to deal with cancer metastases and tumors. Lou said that the company is also looking at PDL-1 with cancer treatment in mind. Additionally, the company intends to tackle sepsis and the resulting cytokine storm it causes, and in addition to TNF, it hopes to use NaNots to hoover up soluble interleukin-1β and interleukin-6 cytokines.
More generally, inhibiting unwanted pro-inflammatory cytokines could potentially be used to address inflammaging, the smoldering background of inflammation present in the majority of older people. NaNotics plans to use its product to target these inflammatory signals, which are secreted by senescent cells and created by other sources. Effectively, NaNots might be used as a form of senotherapeutic.
If the chronic inflammation observed in older people could be effectively reduced without harming healthy cell function, it might delay or even prevent a number of age-related diseases. NaNots are a new and highly selective approach to old problems.
Life Biosciences is bringing partial cellular reprogramming to the clinic
Michael Ringel from Life Biosciences had some interesting news about his company’s work on Yamanaka factors.
The expression of the Yamanaka factors Oct4, Sox2, Klf4, and c-Myc (OSKM) has been found to restore youthful gene expression patterns, reverse epigenetic age, and make old cells and tissues functionally younger.
Life Biosciences has been exploring these so-called reprogramming factors for a number of years, hoping to reverse epigenetic alterations, one of the reasons we age.
It is known that exposing old cells to these factors can make them functionally young again; however, doing so reverts their types to a developmental state. This is a problem because we don’t want the target cells to have their identities erased; heart cells forgetting they are heart cells would be a big problem!
Fortunately, researchers worked out that if cells are only exposed to the Yamanaka factors for just a brief period of time, it is enough to reverse their age without erasing their identities. This is known as partial cellular reprogramming.
The tricky thing is achieving this transient exposure in living animals. Life Biosciences has worked out a way of achieving this short-term exposure.
The company has been using just three of these factors in its research: Oct4, Sox2, and Klf4 (OSK). There was a concern that c-Myc could encourage the onset of cancer, and, as fortune would have it, not using it still allowed partial reprogramming to occur.
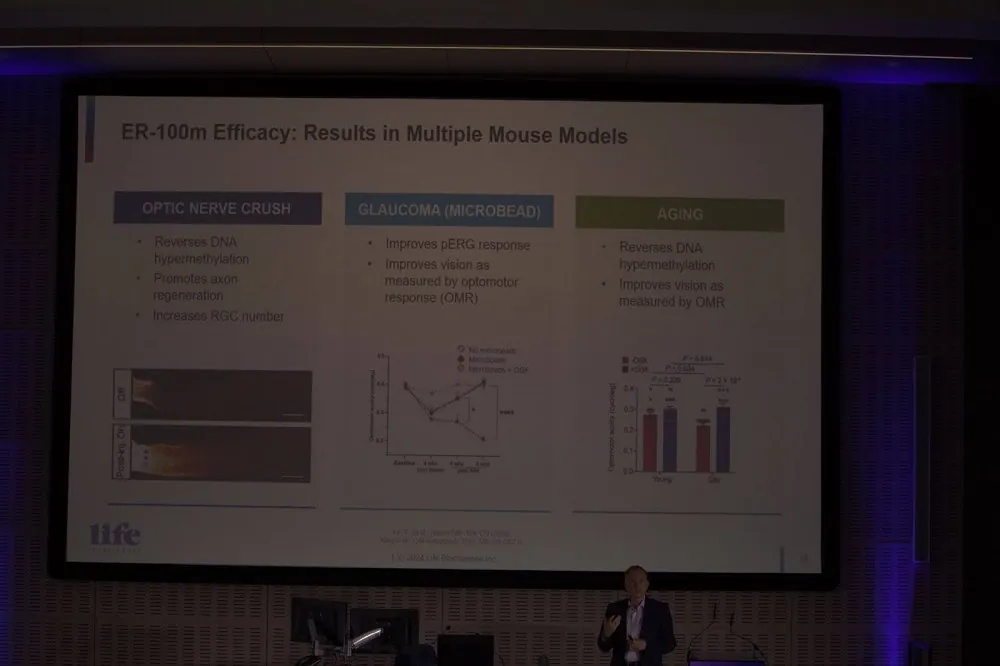
Michael explained that the company is now focusing its efforts on bringing partial cellular reprogramming to the clinic later this year. It is planning to move to Phase 1 for optic neuropathy, particularly glaucoma and non-arteritic anterior ischemic optic neuropathy (NAION).
The company is using doxycycline, something not found in nature, to turn on OSK gene copies delivered to target cells. This allows them to transiently turn the OSK genes on or off based on the presence of doxycycline. Effectively, this acts as a kill switch if things go wrong and controls exposure just enough to partially reprogram the old cells back to being young.
Life Biosciences has demonstrated that this works in mice with age-related optical neuropathy and reports that primate study results have been positive. It seems that partial cellular reprogramming is finally approaching the clinic after decades of research. If successful, it could offer a potential solution to repairing and rejuvenating aged cells and tissues.
Positive results for therapeutic plasma exchange in human trials
Dobri Kiprov from Global Apheresis was on hand to talk about the latest results from his research in therapeutic plasma exchange (TPE). He discussed how his results support that the rejuvenation effect seen in TPE is due to a dilution effect on the negative factors in aged blood, rather than there being some secret sauce in young blood.

He went on to explain that fresh albumin is anti-inflammatory and that replacing the old albumin improves patient outcomes. So far, his company has conducted two human trials for Alzheimer’s using TPE, with the results suggesting the procedure is safe.
The second, larger trial involved 40 people and included sham apheresis. Patients were either hooked up to an apheresis machine to have albumin exchanged or connected to a noise-making machine behind a curtain that did not actually exchange plasma. In this way, the participants had no idea if they were receiving actual TPE or just a mock treatment. This second trial also had two treatment frequencies in the test group.
Dobri reported that grip strength and balance improved in all treatment groups but not in the sham one. This trial used an impressive 35 aging clocks, and, broadly speaking, there was a significant reversal of aging markers. It seems that TPE rejuvenates the stem cell niche and makes the signaling environment more like that seen in younger people.
The take-home here is that the effects of TPE, as demonstrated in mouse studies by people such as Irina and Michael Conboy, appear to translate to humans. This means that TPE could potentially help us to stay healthier and biologically younger for longer.
However, for a rejuvenation technology to be truly successful, it needs to be both cost effective and scalable. As TPE likely needs to be done 3-4 times a year, based on what Dobri suggested, there must be a lot of procedures conducted. As many people would likely want TPE for its anti-aging effects, there is a question of scalability. Clearly, that is a consideration that will require creative solutions.
Perhaps even more intriguing is the possibility we could find out exactly what it is about aged plasma that is harmful and repair it in situ, instead of replacing it with new albumin. NaNots and other new technologies might be harnessed to that effect to achieve this, depending on what needs fixing in the albumin of course. One thing is certain, now we know that TPE works for people, the race is on to find scalable solutions.
A new early type of stem cell
Yuta Lee from Accelerated Biosciences announced that his company has the earliest form of stem cell free from ethical issues. Human trophoblast stem cell (HTSC) stem cells are gathered from ectopic pregnancies, which occur when a fertilized egg implants itself outside of the womb, usually in one of the fallopian tubes.
These stem cells are the earliest stem cells without ethical concerns, as the embryo is non-viable in these pregnancies. They are between embryonic stem cells and mesenchymal stem cells in terms of lineage and potency. Yuta suggested that these HTSC stem cells are also apparently clean of endogenous viruses.
One of the advantages of HTSCs is they are highly scalable due to the number of potential cell passages compared to other types of stem cells.
Yuta reported that HTSC cell secretions, like those of other types of stem cells, inhibit the inflammatory SASP secreted by senescent cells. That suggests that they may find application in the treatment of inflammatory diseases and conditions.
The company has already been successful in its good manufacturing practice (GMP) requirements. GMP describes the minimum standard that a medical manufacturer must meet in their production processes. Accelerated Biosciences wants to work with others to bring solutions for age related diseases.
Cyclarity Therapeutics progressing with human clinical trials
Earlier this year, we interviewed Matthew (Oki) O’Connor after Cyclarity launched human trials to Cure atherosclerosis, and he was at the Summit with an update.

The good news is that the initial part of Cyclarity’s Phase 1 clinical trial in Adelaide is done. The first five dosing levels are complete, and no adverse reactions have been observed with UDP-003.
The next step is about to begin; this will be the ascending dose group. The purpose of this is to gain an initial insight into the pharmacokinetics of a drug’s single dose and its safe dosage range. It is recommended to administer doses to participants one after another, allowing sufficient observation time between each.
Oki also mentioned that Cyclarity is planning for a 150-person trial for Phase 2 in Europe. While there is no date on that yet, they are pushing hard on Phase 1 and so it could even be this year.
Finally, he revealed that his company is developing an AI-based system to optimize cyclodextrin drug development. While the plaques in atherosclerosis are the target of UDP-003, Cyclarity is interested in removing other harmful molecules using this system.
Cyclarity is also working on finding solutions to nanoplastics, things like BPA and PFAS that the company believes its technology could potentially address. This could potentially address unhealthy levels of nanoplastics in people and remove them from the blood, cells, and tissues where they have accumulated.
We are looking forward to hearing more from Cyclarity and are proud that we helped this company to be founded. Heart disease is the number one killer worldwide; to have a solution to treat it effectively would be game-changing.
Our knowledge of female biology and aging is lacking
Jennifer Garrison from the Buck Institute waded in on female biology and aging. She believes that a loss of homeostasis systems in the brain regulates aging. Jennifer highlighted that male biology is better understood than female biology.

She said that females have a shorter healthspan than males. Some of this is likely due to how fast ovaries age, up to twice as fast as other organs. Ovaries appear to be part of a wider signaling system, which isn’t well understood but appears to promote female healthspan and lifespan.
Jennifer talked about how perimenopause causes a breakdown of this communication and promotes aging, and menopause does even more of this.
She said that HRT is a band-aid and can reduce all-cause mortality by up to 30% if given within ten years of menopause. Also, on average, if menopause is later in life, then lifespan is often longer as well. She believes that if we can extend ovarian function by delaying aging of the organ, we could increase female healthspan.
More funding for female aging is urgently needed. Women’s health is seeing research cutbacks by the NIH at a time when it badly needs to be improved. It is more important than ever that more focus is put into understanding how women age and the additional functions ovaries play in that aging.
There are now a number of companies exploring female aging. If their efforts to rejuvenate or extend the healthspan of ovaries succeeds, this will be a great demonstration that aging is not a one-way street.
Lifespan Research Institute: A new org, a new direction
Lifespan Research Institute (LRI) President Keith Comito focused on the always-important need to work together to face the challenges our field presents. Solving aging is the greatest challenge humanity has ever faced, and, as a relatively young industry, it is critical that we collaborate where possible to make rapid progress.

Among the challenges we face is getting wide public support for rejuvenation biotechnology. Keith said, “Bringing the public along with us on the journey is important. We need to meet them where they are, rather than assume they will just get on board.”
Regarding public engagement, he ventured that developing AI-based tools could also help support effective advocacy and determine public sentiment towards rejuvenation biotechnologies. Keith is well known for his work with AI and other disruptive technologies and he feels they could be used to great effect for our field.
He gave four historical examples where interest in rejuvenation appeared to peak:
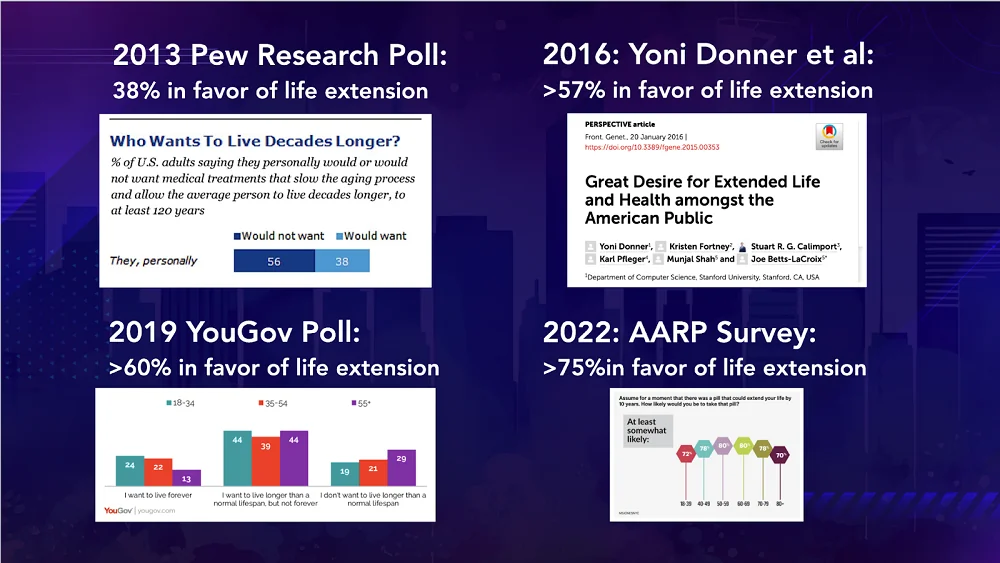
Imagine if we had AI-based tools to help interpret these peaks in public interest and to help identify what approaches work best. This would mean we could more effectively engage with people about the field. We will have more to say about new public advocacy tools in the near future.
Keith also highlighted the importance of non-invasive biomarkers of aging and how machine learning might be a useful tool in our longevity arsenal. He gave an example of when machine learning was used as a detection method for COVID-19.

He suggests that machine learning and AI more generally may be adapted in the context of aging research. For instance, it isn’t hard to imagine how an AI based biomarker system may be useful in the context of functional aging.
Consider a system that could examine gait and body movement and identify trends associated with age-related changes. Combined with clinical biomarkers, these non-invasive biomarkers could potentially help round out more comprehensive biomarker panels.
Finally, Keith took the opportunity to talk about the LRI, our new organization created by the merger of LEAF and SENS in October last year. We have launched a new website that showcases our work and explains how we are adapting to the changing landscape of longevity and rejuvenation research.

LRI has five broad guiding principles behind its research.
- Ability To Boldly Impact the Biology of Aging and Extend Healthy Life
- Capability for Rapid Translation into Humans
- Uniqueness and Non-Duplicative Effort
- Paradigm-Shifting Technological Advances
- Ability to Inspire the Public and Lead the World to Prioritize Aging Research
At our Mountain View, California research center, we have two pioneering labs working on repair-based solutions to age-related diseases.
The Boominathan Lab: Dr. Amutha Boominathan’s research team focuses on exploring mitochondrial biology, creating gene therapies to treat mitochondrial issues, and improving therapies for conditions associated with mitochondrial DNA mutations and aging processes.
The Sharma Lab: Dr. Amit Sharma’s research team is exploring the impact of aging and cellular senescence on immunity with a focus on creating approaches to utilize immune responses for identifying and eliminating senescent cells.
The emphasis is very much on actionable research that helps propel the field forward as rapidly as possible. If you would like to support our non-profit mission for longer and healthier lives, see how you can help us.
See you next year for more longevity and rejuvenation
There were many more talks during the conference, too many to list and discuss here. We have picked out the ones that most resonated with us and our mission of accelerating technologies to overcome age-related disease and extend healthy human lifespan.
The Lifespan team was delighted to be a part of this year’s conference, and we would like to thank our hosts for inviting us. We are looking forward to the 2026 Longevity Summit in Dublin and wish Martin and the team the best in making that happen!