A recent study published in Aging Cell has turned up potential new therapeutics to target the aging extracellular matrix (ECM).
Longevity drug discovery
The discovery of new targets injects fresh lifeblood into the race to develop anti-aging compounds. The strategies used to identify these targets have seen significant innovation recently, with various types of databases and analysis tools being developed over the last decade. These days, longevity drug discovery studies often examined genome-wide data, comparing youthful and older gene expression profiles. Several research groups have cross-referenced these findings with the changes in gene expression that occur when known geroprotectors, such as metformin, NMN, and rapamycin, are administered [1-4]. Those studies have all come to similar conclusions, but researchers at the university ETH Zürich have taken the same strategy a step further by focusing onto the extracellular matrix (ECM) [5].
Could the ECM be overlooked in longevity research?
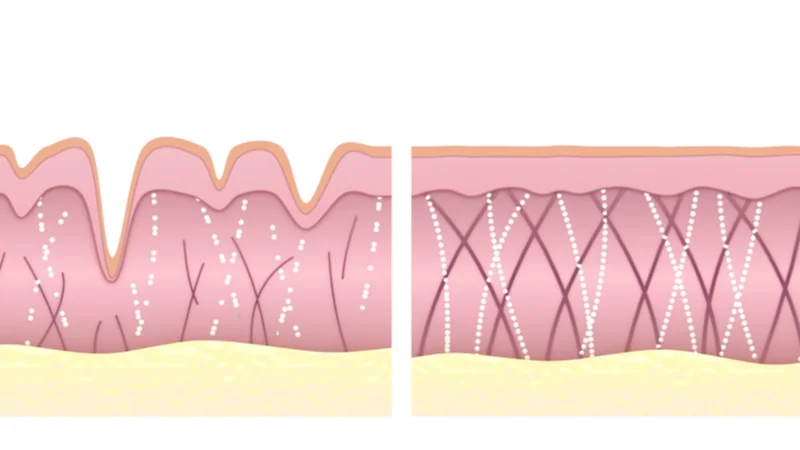
From cancer to high blood pressure to wrinkled skin, ECM modifications are front and center in many age-related diseases. 1027 protein-encoding genes have been implicated in forming, modifying, or remodeling the ECM [6]. Changes in the expression of many of these genes have been implicated as causes of aging, effects of aging, or both. Known geroprotectors have been previously been reported to alter the expression of ECM components, but these mechanisms are rarely considered the main drivers of their therapeutic benefits. The authors hypothesized that these pathways are often overlooked as targets for longevity therapeutics and therefore took advantage of them as the focus of their study.
Mining databases for clues
For the in silico portion of their study, the researchers started with DrugAge and GeroProtectors, databases of compounds that are known to alter lifespan. Cross-referencing these 567 longevity compounds on PubMed, only 16 have published studies reporting on their effects on the ECM. Attempting a different strategy, they then turned to the Connectivity Map database (CMap) on human cell cultures. Here, 41 of 47 longevity compounds significantly altered ECM gene expression, and these compounds represented 10 of the top 12 most differentially expressed ECM profiles. These findings suggest that known geroprotectors may often act through the ECM while also highlighting the lack of published literature on the topic.
Next, the researchers turned to the GTEx dataset to compare the ECM-related gene expression for young vs old human tissues. Their search confirmed previous findings that the expression of collagens decreases with age while matrix proteases increase. However, each tissue type had different ECM gene expression profiles, and the age association also varied between tissues. Because of these differences, their analysis required some tweaking and supplementation with several other published studies. Ultimately, 99 genes were identified whose expression embodies the aging ECM phenotype, which the authors call the “matreotype”.
Using the matreotype to identify missed opportunities
While known geroprotectors helped to identify the matreotype, one of the main goals of the study was to discover novel compounds which modulate both lifespan and ECM. Using this matreotype and the CMap database, 185 compounds were identified, 24 of which have already been shown to increase lifespan in model organisms and 42 of which had previously been predicted, although not proven, to extend lifespan.
The authors sought to verify their in silico results using lifespan and a novel measure of collagen expression in the roundworm model C. elegans. Tretinoin (a retinoic acid receptor agonist) has previously been predicted to extend lifespan, but a single concentration had failed in a large-scale, rapid screening experiment. In this experiment, tretinoin prolonged both collagen expression and lifespan when given at a lower dose to these worms. Experiments into genistein (an isoflavone derived from soybeans) and royal jelly oil (a milky secretion produced by honeybees) also followed a parallel story when optimized for drug delivery methods and concentrations.
Given the functional implication of ECM in healthy aging, we hypothesized that a youthful matreotype might predict drugs promoting healthy aging. Here, we define a youthful human matreotype using data from the Genotype-Tissue Expression (GTEx) project (Consortium, 2013). We query this young matreotype signature with the drug resource Connectivity Map (CMap) (Lamb et al., 2006) data to identify longevity-promoting compounds. We then developed a novel in-vivo tool as a surrogate marker for longevity to find appropriate drug doses to be used for C. elegans‘ lifespan assays. Our results implicate previously known longevity drugs as well as novel drugs, providing a proof-of-concept for our approach.
Conclusions
This study presents a novel strategy and resource for candidate drugs that benefit both ECM and longevity. The newly characterized, age-related matreotype could also be a unique and valuable tool in future research. It successfully predicted a higher number of compounds using fewer possibilities than the strategy that utilized the entire ECM-related genome. However, many of these longevity compounds were associated with the aged, rather than the young, matreotype, leaving many questions regarding its future applicability.
One possibility, the authors suggest, is the different ECM gene expressions between different tissue types. For example, increased collagen expression indicates improved ECM maintenance in tissues like the skin and cartilage, but they are associated with age-related fibrosis in liver and kidneys. Several longevity drugs, for example, impact collagen expression differentially in different tissues, including resveratrol, rapamycin, and genistein [7-9]. Regardless, many compounds that are ripe for further research and development were identified through this strategy.
Literature
[1] Dönertas, H.M., et al. Identifying Potential Ageing-Modulating Drugs In Silico. Trends in Endocrinology & Metabolism (2019). http://dx.doi.org/10.1016/j.tem.2018.11.005
[2] Fuentealba, M., et al. Using the drug-protein interactome to identify anti-ageing compounds for humans. PLOS Computational Biology (2019). https://doi.org/10.1371/journal.pcbi.1006639
[3] Janssens, G.E., et al. Transcriptomics-Based Screening Identifies Pharmacological Inhibition of Hsp90 as a Means to Defer Aging. Cell Reports (2019). http://dx.doi.org/10.1016/j.celrep.2019.03.044
[4] Komljenovic, A., et al. Cross-species functional modules link proteostasis to human normal aging. PLoS Computational Biology (2019). https://doi.org/10.1371/journal.pcbi.1007162
[5] Statzer, C., et al. Youthful and age-related mateotypes predict drugs promoting longevity, Aging Cell (2021). https://doi.org/10.1111/acel.13441
[6] Naba, A., et al. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biology (2016). http://dx.doi.org/10.1016/j.matbio.2015.06.003
[7] Chen, G., et al. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One (2012). https://doi.org/10.1371/journal.pone.0033626
[8] Li, P., et al. Resveratrol inhibits collagen I synthesis by suppressing IGF-1R activation in intestinal fibroblasts. World Journal of Gastroenterology (2014). https://doi.org/10.3748/wjg.v20.i16.4648
[9] Matorit, H., et al. Genistein, a soy phytoestrogen, reverses severe pulmonary Hypertension and prevents right heart failure in rats. Hypertension (2012). https://doi.org/10.1161/HYPERTENSIONAHA.112.191445