Rejuvenating Atherosclerotic Foam Cells
- This drug has already been approved for its first human clinical trial.
According to a study published by Cyclarity Therapeutics, its drug UDP-003 shows benefits in reversing the root cause of atherosclerotic plaques [1].
Fighting the root cause
Many available medications address only disease symptoms in an effort to mitigate them. However, the real challenge is to identify the root cause of a problem and find ways to treat it. This is the approach that Cyclarity Therapeutics is taking with its drug, UDP-003, which targets the cause of atherosclerosis, a cardiovascular disease that is a globally leading cause of mortality and consists of a buildup of plaque in and on the arterial wall.
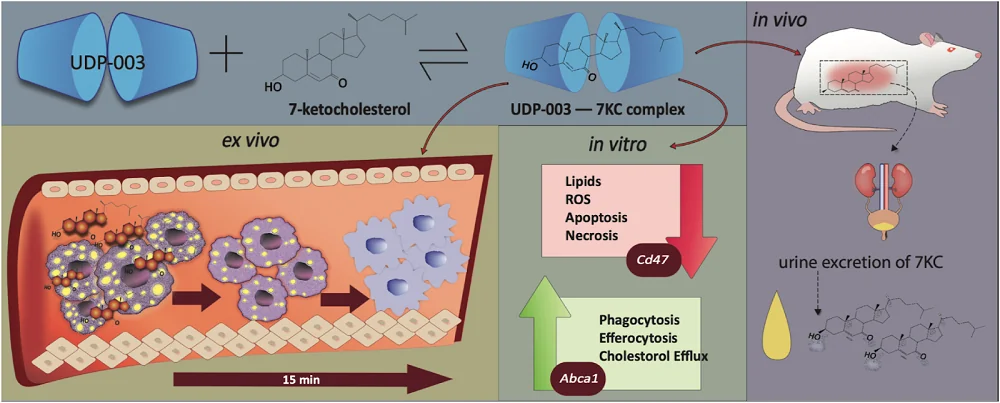
“UDP-003 aims to address a root cause of plaque formation by removing the oxidized cholesterol that accumulates in macrophages,” said Dr. Prerna Bhargava, the lead author of this study. “This buildup transforms macrophages into foam cells. By selectively removing oxidized cholesterol, UDP-003 reverses the foam cell formation and restores the diseased macrophages into healthy cells.” Dr. Bhargava continued.
This is different from current approaches. As Dr. Bhargava explained, “Current treatments- such as statins, PCSK9 inhibitors, ezetimibe, and anti-inflammatory drugs- primarily focus on lowering circulating lipids or reducing systemic inflammation. While these approaches effectively slow the disease progression, they do not resolve the residual vascular inflammation or address the underlying driver of the disease by targeting the buildup of toxic oxysterols.”
From lipids to plaques
Atherosclerotic plaque is built from oxysterols, oxidised low-density lipoproteins (LDL), including the most abundant, highly toxic, and proinflammatory 7-ketocholesterol (7KC).
The pro-inflammatory response is initiated by the accumulation of oxysterols in the arterial walls. This leads to the recruitment of macrophages, which engulf oxysterols, including 7KC; this leads to the transformation of those cells from macrophages into foam cells, which participate in creating atherosclerotic plaques.
Targeting 7KC in foam cells
UDP-003 is intended to stop this chain of events by targeting the buildup of 7KC. UDP-003 is a cyclodextrin, a cyclic glucose oligomer with a hydrophobic cavity and a hydrophilic shell. This family of molecules can form complexes with hydrophobic substances. UDP-003 showed specificity for 7KC, which was ~1000-fold higher than for cholesterol.
The researchers used two experimental treatment modalities: preventative and additive. In the preventative treatment modality, they simultaneously added 7KC and UDP-003 to macrophages for 48 hours. Here, UDP-003 minimized the detrimental effects of 7KC by encapsulating it before it even reaches the cells.
In the additive treatment modality, they first incubated the cells with 7KC for 24 hours. After that, they added UDP-003, and the cells were kept for another 24 hours with both additives. This modality resembles a clinical context in which the treatment would be administered after the arteries were exposed to 7KC and show signs of disease.
Preventable and reversible
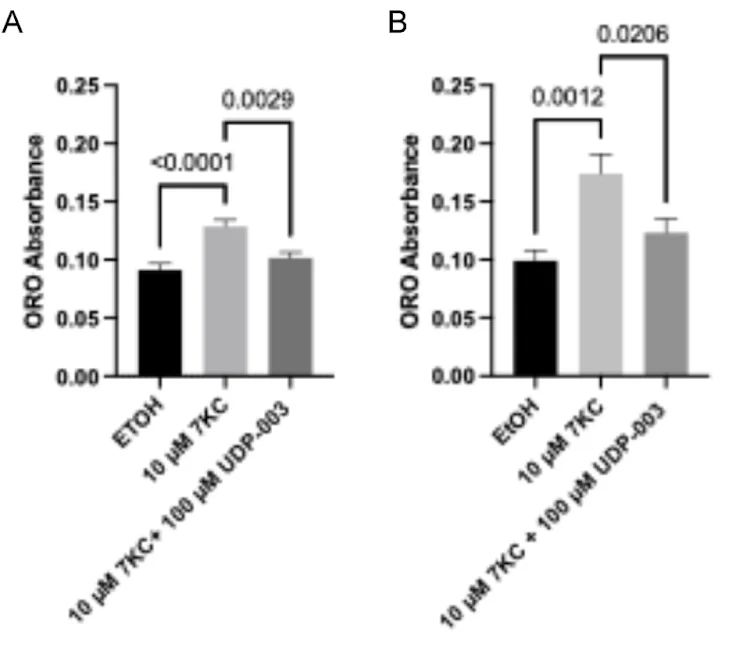
In their initial experiments, the researchers used one of the easiest features of foam cells to capture: lipid droplet accumulation. During the treatment of mouse macrophage cells, the addition of 7KC induced foam cell formation.
Simultaneous addition of 7KC and UDP-003 to the cells resulted in over 20% reduction of lipid droplet marker compared to the cells treated only with 7KC, which, in itself, led to an increase in the lipid droplet accumulation in macrophages. This suggests that UDP-003 can prevent 7KC from entering the cells and causing cellular damage.
In the additive modality experiment, the researchers observed almost a 30% reduction of lipid droplet marker, suggesting that UDP-003 has the ability to reverse the lipid accumulation in cultured macrophages.
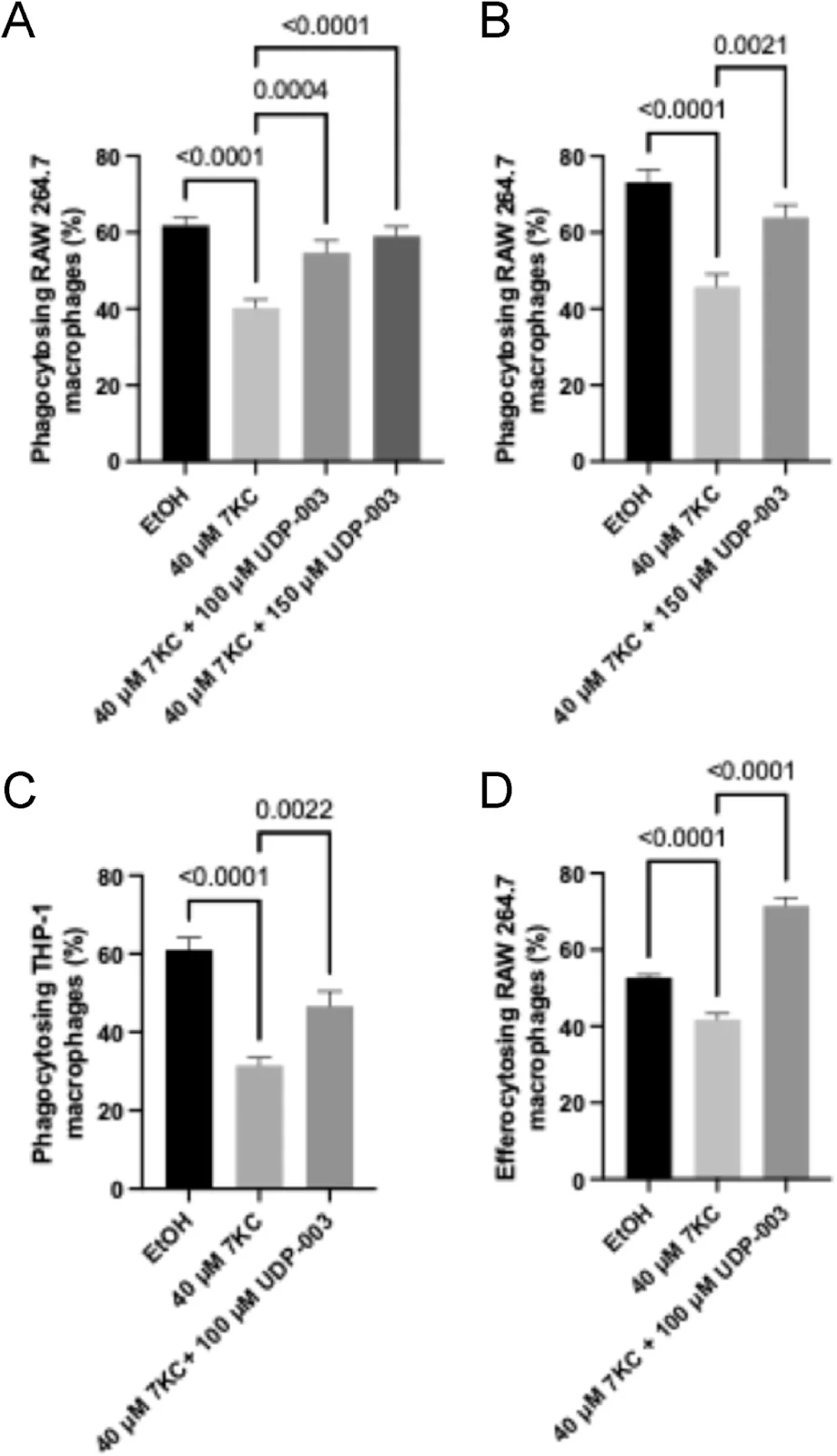
Improving cellular functions
When macrophages turn into foam cells, their cellular functioning suffers. Macrophages engulf pathogens and debris (phagocytosis) and clear apoptotic cells (efferocytosis). Becoming foam cells impairs these functions.
The researchers observed decreased phagocytosis and efferocytosis in 7KC-treated human monocytes and mouse macrophages in both treatment modalities. UDP-003 treatment led to around a 15-20% increase in phagocytosis compared to the cells treated only with 7KC and prevented the loss of efferocytosis caused by 7KC in UDP-003-treated cells in the preventative modality.
Eventually, foam cells undergo cell death by apoptosis or necrosis, with necrosis leading to further tissue damage and contributing to plaque instability by increasing the necrotic core of arteriosclerotic plaque, which consists of dead cells and debris.
Exposing mouse macrophage cells and human monocytes to 7KC led to a substantial increase in the levels of apoptosis and necrosis. UDP-003 treatment reversed and prevented further apoptosis and necrosis by around 40-60%, depending on the treatment modality and cell line.
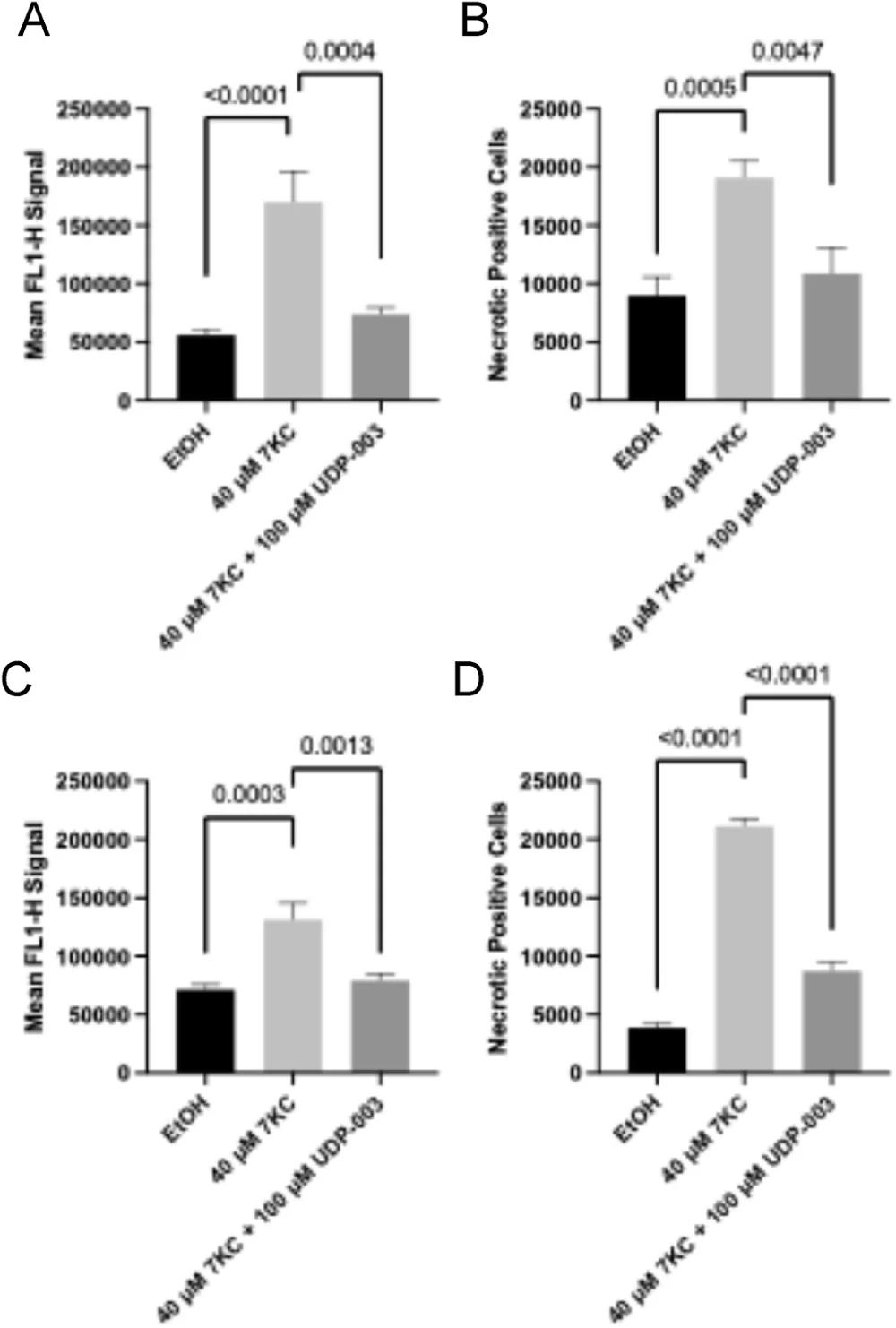
The malfunctioning of foam cells’ molecular processes causes broader systemic issues, such as disruptions in reverse cholesterol transport (RCT), since foam cells are unable to participate in it due to lysosomal dysfunction. This disrupts the first step of RCT: the removal of cholesterol from cells (cholesterol efflux).
Measuring the cholesterol efflux in murine macrophage cells exposed to 7KC showed that while 7KC reduced cholesterol efflux, UDP-003 reversed the reduction caused by 7KC, suggesting atheroprotective functions.
Fighting inflammation
Inflammatory responses were explored at the levels of gene expression in mouse macrophage foam cells. 7KC led to an increase in the expression of genes playing a role in immune cell recruitment and inflammation along with Nf-κB, which plays an essential role in the modulation of immunoregulatory gene expression. UDP-003 treatment in the additive modality decreased the Nf-κb and some of the inflammation-related gene expression. While this effect was not statistically significant in the preventative modality, a similar trend was observed.
UDP-003 treatment also significantly reduced ROS levels induced by the 7KC treatment in both treatment modalities in mouse macrophage cells. The researchers suggest that decreasing levels of ROS can help to close the inflammation feedback loop driven by 7KC, as lower ROS levels could reduce the amount of cholesterol that is being oxidised into 7KC.
Putting it into context
While cell culture experiments can provide a plethora of valuable information, atherosclerotic foam cells are located in the plaques within the arterial wall, and it’s essential to know whether UDP-003 treatment can be effective in such a context. To test this, the researchers used human arterial tissue containing atherosclerotic plaque and treated it with UDP-003. Those experiments have shown the UDP-003 potential for 7KC solubilization and removal from the human plaque.
Limited animal models
Cell culture experiments were followed by experiments in living organisms. Experiments in rats showed that UDP-003 treatment leads to 7KC being excreted in the urine during the first four hours of UDP-003 administration.
However, the results of the experiments in three different atherosclerotic mouse models were less satisfactory. The results indicated a trend towards lower 7KC levels in plasma and whole blood in female but not male mice. However, these results were mostly not statistically significant. Similarly, there was no visible impact of UDP-003 treatment on the lesions in the part of the aorta that connects to the heart (the aortic root) and minimal impact of the treatment on plaque reduction in the aortic root.
Dr. Bhargava shed some light on those results and the animal models’ limitations: “Unfortunately, there are currently no animal models that fully replicate human atherosclerosis. Most available models primarily evaluate hypercholesterolemia rather than the complex pathology of plaque development and progression. While LDLR-/- and ApoE-/- knockout mice are commonly used as potential models for atherosclerosis because they promote plaque accumulation in mice, these models have limitations. In humans, LDLR and ApoE receptors remain functional even in the presence of significant plaque buildup. Therefore, drawing parallels between the two is a poor comparison. Although high cholesterol can contribute to the development of plaque, the prevailing models are not a complete representation of atherosclerosis.“
Dr. Bhargava adds that “genetically modified animal models provide partial insights and cannot fully predict human outcomes.” Still, this study used multiple experimental approaches and models to evaluate the drug’s efficacy, and mouse models are just part of the picture.
Clinical trials underway
This is not the first time we have reported on Cyclarity’s research into UDP-003. Earlier this year, we reported that this drug had already received regulatory approval to begin its first in-human clinical trial. “This study is part of the package submitted for clinical trials approval,” Dr. Bhargava explained.
Given that things will go as planned, the quickest time we can expect the full approval would be 2030, according to what Dr. Matthew O’Connor, CEO of Scientific Affairs at Cyclarity Therapeutics, told us in a recent interview. The company is interested in pursuing accelerated and adaptive approaches “to bring our therapy to people as soon as it’s ready.”
Beyond atherosclerosis
Dr. Bhargava summarized, “Our research has demonstrated that UDP-003 removes oxidized cholesterol from dysfunctional macrophages, restoring them to a healthier phenotype, and improving their cellular function.”
“These cumulative findings provide strong evidence that our candidate drug has the potential of targeting a root cause of atherosclerosis, in contrast to current approaches that primarily manage disease symptoms rather than addressing the underlying pathology. UDP-003 is a first-of-its-kind drug with the potential to prevent atherosclerotic disease progression by selectively targeting oxidized cholesterol.”
“Oxidized cholesterol toxicity has been implicated in numerous diseases including Alzheimer’s disease, Niemann Pick disease, age-related macular degeneration, and non-alcoholic fatty liver disease,” Dr. Bhargava informed us. “We aim to expand our work to evaluate UDP-003’s potential against these diseases where oxidized cholesterol toxicity can play a prominent role.”
Disclaimer: Dr. Matthew O’ Connor, who is the corresponding author of the study, was previously the Vice President of Research at the SENS Research Foundation for nine years (now LRI, of which Lifespan.io is part). The initial research into the UDP-003 compound was conducted while Dr. O’Connor worked for SENS.
Literature
[1] Bhargava, P., Dinh, D., Teramayi, F., Silberg, A., Petler, N., Anderson, A. M., Clemens, D. M., & O’Connor, M. S. (2023). Selective Removal of 7KC by a Novel Atherosclerosis Therapeutic Candidate Reverts Foam Cells to a Macrophage-like Phenotype. bioRxiv : the preprint server for biology, 2023.10.23.563623.