Many people dread the thought of getting old, not because getting older is anything to be ashamed of but because of the age-related diseases that go with it. Aging is inevitable, right? No, it isn’t. In fact, some animals do not age at all. Join us as we explore the fascinating world of animals that defy aging!
Do we have to age?
Aging is the leading cause of death and suffering worldwide. Cancer, diabetes, heart disease, Alzheimer’s disease, Parkinson’s disease, and many more problems are all caused by aging. These diseases cause a great deal of suffering and a loss of independence, and they will ultimately kill you if nothing else does first. That makes slowing down or even reversing aging to delay their arrival an attractive prospect.
Unfortunately, we are taught to accept that aging is inevitable. We are encouraged to accept that there is nothing we can do about it and that our minds and bodies will begin to fail. Society tries to convince us the rising risk of age-related diseases is just a part of life and something we should just accept. For much of history, that has been true.
This idea is so deeply ingrained in our culture and society that we rarely stop and ask: do we have to age? The good news is that there is no reason why humans could not enjoy considerably increased healthy longevity if the appropriate technologies are developed.
This isn’t some cheesy sales pitch from a snake oil salesman offering you an elixir of youth. We are a non-profit organization, and we are not here to sell you anything. However, we would like to explain why aging is not inevitable and show some real-world examples of animals that show it isn’t.
When it comes to immortality, what you mean is important
First, let’s talk about the elephant in the room. In our line of work we are often asked “Could humans become immortal?” The answer depends on how, exactly, you define the term. If you define it as living forever and being unkillable like in a comic book or movie, then, no, it is highly unlikely.
However, if you define it in terms of showing no decline in survival characteristics, no increase in disease incidence, and no increase in mortality with advancing age, then that may indeed be possible.
This is far from a matter of semantics. The first idea is science fiction, but the second is based on real-world biology that evolution has already selected for in certain very long-lived species.
Some species live very long lives
We humans live quite a long time compared to many other animals, but some animals make our lifespans look like rookie numbers.
| Species | Recorded lifespan |
| Rougheye rockfish | 205 years [1-2] |
| Aldabra Giant Tortoise | 255 years |
| Sea anemones | 60–80 years |
| Freshwater pearl mussel | 210–250 years [3] |
| Ocean Quahog clam | 507 years [4] |
| Greenland Shark | 400 years |
These figures are based on the limited observations available. It may well be the case that there are older examples of these species that have not been confirmed.
Senescence and negligible senescence
This is impressive stuff, but there are a few species that turn the idea of inevitable aging upside down. Some animals are negligibly senescent (NS): they do not appear to age. An organism is considered NS if it does not show any loss of survival characteristics, such as strength, mobility, and senses; an increased mortality rate; or a loss of reproductive capability with age.
The following animals have been reported to fit all or most of these criteria. Mortality rates for humans and most animals increase dramatically with age beyond reaching reproductive maturity, but it is not so for these creatures.
| Species | Recorded lifespan |
| Lobsters | 100+ years |
| Naked mole rat (Heterocephalus glaberis) | 28 years |
| Freshwater Hydra (Hydra vulgaris) | Biologically immortal |
| Lake sturgeon (Acipenser fulvescens) | 152 years (Presumed NS) |
| Clams such as Panopea generosa | 160 years (Presumed NS) |
Lobsters don’t age like we do
 Some species of lobster do not appear to age. They remain active and continue to grow and reproduce just like young lobsters do. For these lucky lobsters, life goes on as normal without the concerns of becoming weaker and dying from age-related diseases.
Some species of lobster do not appear to age. They remain active and continue to grow and reproduce just like young lobsters do. For these lucky lobsters, life goes on as normal without the concerns of becoming weaker and dying from age-related diseases.
The secret of their success appears to be how great they are at repairing their DNA. This makes them much more resistant to cancer and the debilitating effects of old age in general.
Just like us, lobster cells have chromosomes, long DNA molecules containing some or all of their genetic material. The chromosomes are protected by special caps called telomeres made from repeating DNA sequences. As our cells divide and we age, these caps erode until there is nothing left; at that point, the cell stops dividing and dies. This loss of telomeres is one of the hallmarks of aging.
However, the lobster can get around this problem because its cells have access to an enzyme called telomerase. This enzyme allows their cells to replace lost telomeres by topping up the protective cap each time they divide. In this way, the lobster can just keep on making new cells with no fear of running out.
We also have telomerase, but in most of our cells, access to it is turned off. This is thought to be an anti-cancer safety measure, as cancer cells hijack telomerase to become immortal and divide non-stop. Somehow, the lobster has managed to not only turn telomerase on but also found a way to avoid that cancer risk.
Naked mole rats do not seem to age
 Naked mole rats are one of the most commonly studied organisms in longevity research, and for a good reason. These animals can live for over years, which is massive compared to most other rodents, which only live 2-3 years.
Naked mole rats are one of the most commonly studied organisms in longevity research, and for a good reason. These animals can live for over years, which is massive compared to most other rodents, which only live 2-3 years.
They do not experience age the same as we do; they remain active, strong, and fertile for their entire adult lives. For us, the risk of us dying doubles every 8 years after age 40, but the naked mole rat sees no increase in that risk, no matter how old it is!
This is likely because naked mole rats have superior DNA repair systems. This keeps their genome protected from mutations and reduces their cancer risk.
Some researchers are trying to find out if the longevity of the naked mole rat might be exported to humans, and scientists have successfully transferred a longevity mechanism from naked mole rats to mice [5]. This opens the door for potentially doing the same for humans. One day, we might be able to harness the superior DNA repair of the naked mole rat and significantly reduce the impact of aging to our bodies.
The Hydra is biologically immortal
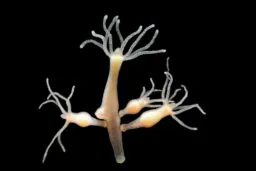 If those animals are not weird enough, the hydra is an animal that is basically biologically immortal. Named after the sea monster from Greek mythology, this tiny water creature can regenerate just like its mythological namesake.
If those animals are not weird enough, the hydra is an animal that is basically biologically immortal. Named after the sea monster from Greek mythology, this tiny water creature can regenerate just like its mythological namesake.
It is able to regenerate itself entirely, even if it is chopped into tiny pieces. Within days, a small piece of hydra can become a complete animal! Barring predation and changes to its environment, it is one of the few species for which the phrase “biological immortality” would be appropriate [6].
The hydra seems to achieve this feat of regeneration thanks to its stem cells. It is able to replace the entirety of its cells every few weeks, which allows it to remove those that are damaged or old.
There have been a number of studies showing that the hydra does not appear to age and, if kept in ideal conditions, can continue living indefinitely. Its chances of dying with each passing year do not increase as occurs in humans, and its fertility remains high regardless of age.
The hydra is quite unique in how its cells work, and it is quite unlike the majority of other organisms on the planet; it is a true oddball but fascinating all the same.
Negligible senescence does not mean they cannot die
It is worth noting that even though a number of species enjoy negligible senescence and either do not age or age immeasurably slowly, they are still vulnerable to predation, accidents, starvation, environmental dangers, changes to their environmental niches, and diseases. This means that extremely old examples of these species with negligible senescence are very rare, especially in the wild.
To further complicate matters, we often need to sacrifice the animal in order to measure its age by examining the deep tissues and marks inside bones, much like measuring rings in a tree trunk.
This means that we cannot know the maximum age that might be achieved by these species, so the above numbers are based on what information we have; there could well be considerably older examples out there. However, it is clear that species with negligible senescence do not deteriorate with age and may live considerably longer than has been recorded.
Can we achieve negligible senescence?
All of this is great news if you happen to be a lobster and can avoid the fisherman’s pot long enough to reach a ripe old age, but what about us; how can we benefit from the same advantages that negligibly senescent species do?
It is clear that we would have to wait a long time, perhaps forever, before evolution selected the same traits in humans, so something a little more direct is needed. Some scientists propose that we can engineer negligible senescence by using a repair-based approach to the damage that aging causes.
Other researchers have built on the original concept of SENS, and in 2013, the Hallmarks of Aging was published. This landmark paper broke aging down into nine distinct processes known as hallmarks and essentially gave researchers a way to classify aging and an insight into what processes they might target to slow down or even reverse aging.
While there are quite a number of aging theories, the Hallmarks of Aging appears to be one of the more popular, judging by how many times it has been cited and how often it is used in academia. Essentially, the Hallmarks have given researchers a list of targets to develop therapies for, and now the race is on to create them.
Should negligible senescence be achieved in humans through SENS or other approaches such as partial cellular reprogramming, it would potentially mean the end of age-related diseases and ill health, a most worthy goal indeed.
No, aging is not inevitable
Back in 2017, a great deal of fuss was made about humans achieving negligible senescence, with a number of articles suggesting that it is impossible. The reason is that poorly informed media organizations interpreted a research paper very badly, assuming that the authors imply that because aging is inevitable, we cannot do anything about it [7].
The media was filled with articles smugly proclaiming in some cases that aging is unstoppable and mathematically impossible to defeat. The problem with this interpretation is that it is just plain wrong. The original paper is, strictly speaking, correct in that aging damage is indeed inevitable, but it makes no assumptions about interventions. The publication says a great deal about what evolution has done and is likely to do based on observation, but that says absolutely nothing about what medicine may achieve in the future.
One cannot apply such thinking when it comes to engineering negligible senescence in humans through the periodic repair of age-related damage. So, quite simply, publications like this make little difference to work in this field, and they change the plausibility of us achieving negligible senescence in no way whatsoever.
A more recent example in 2021, which was again met with an almost gleeful declaration by the press that aging cannot be stopped, was the ‘invariant rate of ageing’ paper [8]. Unfortunately, once again, the reporting was based on a similar misunderstanding of what the study actually said.
The study was actually not a study about longevity or that aging was inevitable. It was trying to understand what influences the rate of aging across species, determining how much is the result of evolved biological processes and how much is due to the effects of the environment. While the research itself has obvious merit, scientifically speaking, far too many outlets chose to represent it in a different light.
The irony is that instead of showing that aging is indeed inevitable, the research instead shows that eventually humanity will run out of ways in which environmental improvements will increase our lifespans. At that point further gains will only be achieved through medical interventions that address the aging processes directly and either repair the damage aging does, or slow aging down by making us more resilient.
Can we learn how to live longer, healthier lives from these animals?
There is a clear difference between Hollywood-style immortality and negligible senescence, with the latter being a plausible goal in the next few decades. Evolution has already demonstrated that negligible senescence is indeed possible; now, the next big challenge is to use an engineering approach to aging to see if we can emulate in people what nature has done in a few lucky species.
Although it is clear that some animals live for a very long time, it is still unknown if we can apply that to people. Some researchers are trying to find out if the longevity of some animal species such as the naked mole rat might be transferred to humans.
The good news is, we have a list of therapeutic targets and a far better understanding of what aging is than we did even 10 years ago, and there is a lot more interest in tackling aging from both the academic and investment communities.
If you are interested in seeing how progress is going towards that goal, check out the Rejuvenation Roadmap, our curated database tracking aging research.
Literature
[1] Munk, K. M. (2001). Maximum ages of groundfishes in waters off Alaska and British Columbia and considerations of age determination. Alaska Fish. Res. Bull, 8(1), 12-21.
[2] Cailliet, G. M., Andrews, A. H., Burton, E. J., Watters, D. L., Kline, D. E., & Ferry-Graham, L. A. (2001). Age determination and validation studies of marine fishes: do deep-dwellers live longer?. Experimental gerontology, 36(4), 739-764.
[3] Ziuganov, V., Miguel, E. S., Neves, R. J., Longa, A., Fernández, C., Amaro, R., … & Johnson, T. (2000). Life span variation of the freshwater pearl shell: a model species for testing longevity mechanisms in animals. AMBIO: A Journal of the Human Environment, 29(2), 102-105.
[4] Munro, D., & Blier, P. U. (2012). The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging cell, 11(5), 845-855.
[5] Zhang, Z., Tian, X., Lu, J. Y., Boit, K., Ablaeva, J., Zakusilo, F. T., … & Gorbunova, V. (2023). Increased hyaluronan by naked mole-rat Has2 improves healthspan in mice. bioRxiv, 2023-05.
[6] Martı́nez, D. E. (1998). Mortality patterns suggest lack of senescence in hydra. Experimental gerontology, 33(3), 217-225.
[7] Nelson, P., & Masel, J. (2017). Intercellular competition and the inevitability of multicellular aging. Proceedings of the National Academy of Sciences, 201618854.
[8] Colchero, F. et al. The long lives of primates and the ‘invariant rate of ageing’ hypothesis. Nature Communications (2021), doi: 10.1038/s41467-021-23894-3



