Female-Specific Mechanism of Neurodegeneration Discovered
- A heavily expressed gene leads to neuropathology.

- A gene on the X chromosome, KDM6A, is more heavily expressed in women than men.
- Silencing this gene in the microglia of a mouse model had benefits against multiple sclerosis.
- Metformin was also found to have related benefits in mice.
Scientists have found that a gene on the X chromosome, which escapes silencing, promotes inflammation and neurodegeneration in a mouse model of multiple sclerosis, but the effects can be reversed with metformin. Human RNA sequencing data supports these findings [1].
Why are women more vulnerable?
Multiple sclerosis (MS) is an autoimmune and neurodegenerative disease that is most commonly diagnosed between the ages of 20 and 40. In MS, circulating immune cells, specifically lymphocytes and macrophages, infiltrate the central nervous system, causing various kinds of damage, such as a loss of myelin and the activation of microglia and astrocytes, the brain’s supporting cells. The resulting neuroinflammation, a common feature of MS and other age-related neurodegenerative diseases such as Alzheimer’s, is thought to be a major culprit [2].
Interestingly, females are more susceptible to MS, with a ratio of 3:1 compared to males [3], another aspect in which it resembles Alzheimer’s. Women are also more prone to relapses. The sex differences in the prevalence and development of these diseases have baffled researchers for decades. A new study from UCLA, published in Science Translational Medicine, yields interesting clues and a possible therapy.
An X chromosome gene that evades silencing
According to the paper, “sex differences in microglia can be caused by differential effects of sex hormones (estrogen versus testosterone), sex chromosomes (XX versus XY), or both.” Since males only have one X chromosome, the expression of the genes sitting on two chromosomes should be twice as high in women than in men. This is solved by the random inactivation of one X chromosome in females, but some genes escape silencing.
One such gene is Kdm6a (lysine demethylase 6A). Previous research has found that it has higher expression in females compared to males. Kdm6a encodes a protein which derepresses a particular region of chromatin via histone demethylation, allowing the translation of resident genes.
The researchers first studied brain-resident macrophages (microglia) derived from humans and detected high levels of Kdm6a, especially in cells from female donors. They then knocked out Kdm6a and observed a loss of its function in the cells.
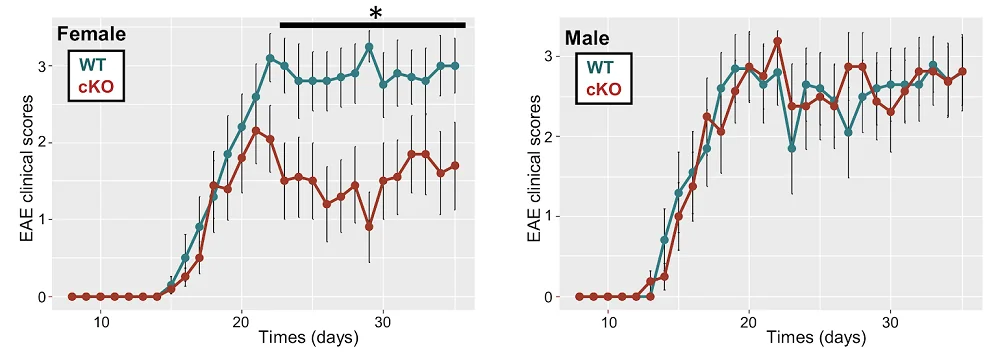
The team then moved to an established mouse model of MS and again knocked out Kdm6a specifically in microglia. Female conditional knockout (cKO) mice exhibited much lower disease scores. Neuropathological assessments showed decreased axonal damage markers (APP) and increased intact axon markers (NF200) in cKO mice. More myelin was observed in cKO mice, indicating neuroprotective effects.
Kdm6a deletion in microglia also altered gene expression profiles. In healthy cKO mice, disease-associated microglial markers were indistinguishable from wild type mice. However, in MS cKO mice, resting microglial markers were slightly increased, suggesting a shift from disease-associated to resting microglial states. Gene ontology enrichment analysis indicated that several downregulated genes were involved in inflammatory responses.
The researchers repeated some of the experiments using both male and female mice. As expected, while female cKO mice received significant protective effects, males did not.
“Sex chromosomes and sex hormones achieve a balance through evolution,” said study lead author Dr. Rhonda Voskuhl, director of the Multiple Sclerosis Program at UCLA Health and lead neurologist for the UCLA Comprehensive Menopause Program. “There is a selection bias to do so. Females have a balance between X chromosome-driven inflammation that can be good to fight infections at child-bearing ages. This is held in check by estrogen, which is anti-inflammatory and neuroprotective. As women age, menopause causes loss of estrogen, unleashing the proinflammatory and neurodegenerative effects of this X chromosome in brain immune cells.”
Metformin provides protection
The researchers then gave mice metformin, a drug known in the longevity field for having possible geroprotective effects. Metformin is an anti-diabetes medication, whose mechanism of action is not fully understood, but it is thought to work via multiple pathways. One of its effects, which rarely gets attention, is to inhibit the histone demethylase activity of KDM6A.
Following this treatment, disease clinical scores improved in metformin-treated mice compared to vehicle-treated controls. Metformin reduced microglial activation and increased resting microglial markers. Gene expression changes mirrored those observed with Kdm6a deletion, indicating a reversal of disease-induced changes.
Due to metformin’s multiple mechanisms of action, it’s hard to ascribe the benefits solely to KDM6A inhibition. However, the treatment benefited almost exclusively females except for some limited early-disease benefits in males. This sex-specific effect is consistent with the KDM6A route.
Finally, the researchers examined the expression of KDM6A using human RNA sequencing data on people with MS. They found that in women, KDM6A expression was higher, and more microglial genes were dysregulated, supporting the findings in mouse models.
“It has long been known that there are sex differences in the brain,” said Voskuhl. “These can impact both health and neurological diseases. Multiple sclerosis and Alzheimer’s disease each affect women more often than men, about two to three times as often. Also, two-thirds of healthy women have ‘brain fog’ during menopause. These new findings explain why and point to a new treatment to target this.”
Voskuhl added that together, these findings may support the use of estrogens that target the brain to keep its balance, and thereby protect it, during menopause.
Literature
[1] Yuichiro Itoh et al. Deletion of the X-chromosomal gene Kdm6a in microglia of female mice ameliorates neuroinflammation and restores translatome profiles. (2025) Sci. Transl. Med.17,eadq3401.
[2] Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., … & Kummer, M. P. (2015). Neuroinflammation in Alzheimer’s disease. The Lancet Neurology, 14(4), 388-405.
[3] Portaccio, E., Magyari, M., Havrdova, E. K., Ruet, A., Brochet, B., Scalfari, A., … & Amato, M. P. (2024). Multiple sclerosis: emerging epidemiological trends and redefining the clinical course. The Lancet Regional Health–Europe, 44.








