Dr. Andrea Maier, Oon Chiew Seng Professor in Medicine at the National University of Singapore, is a veteran geroscientist and one of the most familiar faces in the rising field of longevity medicine, which aims to translate the early successes of geroscience into clinical practice. Parallel to her scientific career, Andrea runs her own longevity medicine company, Chi Longevity, and is the founding president of the Healthy Longevity Medicine Society.
However, where others are perfectly happy providing lucrative concierge services to wealthy customers, Andrea has been working on bringing longevity medicine to the masses. A few days ago, NUS announced the launch of the Clinical Trial Centre of the NUS Academy for Healthy Longevity “to enhance research capabilities and accelerate the clinical translation of geroscience into real-world solutions,” and we decided it was a great moment to catch up with Andrea and talk about precision geromedicine and longevity for all.
What was your personal journey to the longevity field, and what does human longevity mean to you?
I love the human body, as well as those of animals and plants. I love creatures – everything that grows and has the capacity to rejuvenate. I think I am simply in love with the biology of aging. That’s why I really want to maintain whatever has been built, understand why it’s deteriorating, and what consequences that has.
In terms of my journey, I was always good at biology and mathematics. Don’t ask me to speak multiple languages, though. I think I finished high school with the lowest possible marks in Latin and Greek. As you know, I studied medicine. I love being an internal medicine specialist and geriatrician, but psychologically, I’m more of a surgeon. I love making decisions, I love the knife, I love action. So, I think I’m a mixture of loving biology, making decisions, and being a bit of a politician.
I didn’t know that. Did you have a career as a surgeon?
No, but as a student, I did three different jobs to finance my medical studies. One was working night shifts in a bar – I’m very good at making cocktails. The second, if any of the readers drives an old VW Passat, the back of the car might have been built by me. I worked in the car industry for a long time to earn money. And the third was helping out in the surgical departments on Saturdays and Sundays, especially in the operating room.
I saw many appendectomies and colectomies, and I loved working with my hands in people’s abdomens. I discovered that surgery is wonderful because you can touch the organs, and it’s a sort of art. However, it’s also very repetitive. I love to work with my hands, but not with that much repetition.
So, like some other people in the field, you were trained as an MD but ended up doing geroscience.
When I was in my second year of medical studies, I started doing research on monocytes, macrophages, and lung diseases because I wanted to discover why people get COPD. Then, in my third and fourth years, I did research on cytomegalovirus (CMV) and aging in Groningen, so I was already moving between countries. From the beginning of my medical studies, I was involved in research, and from the third or fourth year on, I was specifically involved in aging research.
Later, I did research in a psychiatric medical units and started to study traditional Chinese medicine. I also went to China to study further, and I think that’s where it all clicked. I remember one morning at 4:00 AM – far too early – when I had to do Tai Chi with a very old lady. She seemed very young to me, although she was over 90 years old. And I thought, “I want to discover why.” I can still see her in front of me. I wanted to understand why she was so healthy, lean, and flexible. And I decided then that I wanted to do geriatrics, because at that time, we thought geriatrics was the aging field.
I still see myself in 1999, trying to write an email from a small town in China to Groningen, which was the place to do geriatrics at the time, to see if I could do research with them. They accepted me. So, I’m a bit of a dinosaur in this field.
That’s an amazing story. Are you still practicing Tai Chi?
I was sort of forced to do Tai Chi every morning, but if you give me the opportunity, I’ll choose kickboxing. I’m much better at that. So, it’s absolutely not for me, but I do see its strengths – the balance, the relaxation component. It’s quite rewarding. But I prefer kickboxing.
Today, you are at the National University of Singapore (NUS), which has become a huge longevity hub. How did you get there, and what is Singapore’s place in the current longevity landscape?
I first went to Melbourne. While I was already considering that move, because I got a headhunting offer to manage the non-surgical part of the Royal Melbourne Hospital and lead the internal medicine research at the University of Melbourne, Singapore also knocked on my door. I initially said no because I had signed my contract in Melbourne.
But the attractiveness of Singapore was its real dedication to making healthy longevity work. There’s a dedication to change, to having a long-term impact, and to bringing evidence-based science and clinical care to the table. This is driven by the fact that Singapore will have a super-aged population in the coming years. There’s a recognized need to change, and there’s a long-term vision, which makes it very attractive.
Of course, it’s also a hub in the APAC region. If you want to start public-private partnerships, it’s much easier here than in Australia. Asia is the new hub for longevity investment, and Singapore is partly driving that.
The university is superb, great partnerships are already in place, and – most importantly – there is a driver for the entire field, which is the dean, Professor Chong Yap Seng. He is an unbelievable driver of this ecosystem. Twelve years ago, he was already thinking about what we now call healthy longevity medicine and how to make it happen.
He was revolutionary, studying children even before they were born to understand the early determinants of aging. This is all captured in the “human potential” programs. Human potential applies to every age group, and I now use that term very often. We’re talking about fulfilling human potential at every life stage.
Maximizing longevity as fulfilling human potential, I like it. Is the university the central player, or is there also government support, like in Saudi Arabia?
Oh yes, the government is hugely invested. A couple of years ago, the government launched “Healthier SG” [Healthier Singapore]. This program is embedded in the public health structure, where every Singaporean has a primary family physician. That GP is tasked with bringing preventative care into that individual’s life. It’s a huge initiative.
We are starting rigorous, science-based screening programs from the age of 40 onwards. But it’s not just about screening; it’s about helping families make lifestyle changes and focusing on the early detection and prevention of age-related diseases.
What we see now is a fusion of these ideas. You have a strong public health sector, we are discovering how to apply healthy longevity medicine in clinical practice, and now we’re investigating how to merge the two. We are figuring out how to bring the academic thought of healthy longevity medicine into the public health sector. That’s the stage we are at right now.
That brings us to the new Centre for Healthy Longevity Clinical Trials. The press release says its purpose is to “accelerate precision geroscience medicine research.” What does that mean?
Healthy longevity medicine is the public-facing term. Precision geroscience medicine is the academic term we use in the specialized field. Healthy longevity medicine is defined as optimizing health and healthspan by targeting aging processes across the lifespan. Precision geroscience medicine is simply precision medicine for the aging field.
Everyone now knows what precision medicine is – it’s taking individual characteristics into account, looking at the individual level rather than the group level. In oncology, we have precision oncology. In endocrinology, adapting insulin levels based on a continuous glucose monitor is precision medicine.
My colleague Guido Kroemer and I had many conversations about how we should term our field, and we came up with “precision geromedicine.” We are also introducing terms like “gerodiagnostics” and “gerotherapeutics” because we need our own grown-up vocabulary that everyone understands.
To establish this field, we need trials. That’s why we started the NUS Academy for Healthy Longevity Clinical Trials Unit. We’ve been operational for about four years and have already finished trials, but we didn’t have the capacity to run parallel trials and truly grow the space. Now we do.
We have developed standardized operating procedures for measuring things like women’s health, oral health, and cognition, especially for middle-aged individuals. This is a sector where we often lack the necessary diagnostic tools; everything either comes from geriatrics, where you have ceiling and flooring effects, or from primary care, where tools lack sensitivity.
We have established biological, clinical, and digital biomarkers of aging, and we are matchmaking them with therapeutics in both unimodal and multimodal interventions. Think about nutraceutical trials, drug trials, or combinations of lifestyle with nutraceuticals. This is what our field needs. We know one supplement or one drug might work, but that’s not real life. In real life, people do several things, and the effects might depend on sleep or physical exercise. We now have the capacity and infrastructure to study that.
Multimodal trials are tricky, and you also have the challenge of finding good endpoints and biomarkers. What is your solution?
It’s actually not that hard. I think we are in a space where we can allow ourselves to try and to make errors. We are toddlers in this field, not yet grown-ups. We don’t know what really works yet.
So, we take a combined de-risked and high-risk approach. Our trials always have primary outcomes that are known by the FDA. This could be something like VO2 max, where we know its predictive value for long-term outcomes, or HbA1c, a marker everyone understands. We borrow sophisticated clinical or biological parameters from other fields where I, as the principal investigator, know how hard or easy it is to change them.
We mix that conventional trial design with innovative elements. For example, in our ABLE study, we tested calcium alpha-ketoglutarate (AKG) for six months. We only included 40- to 60-year-olds whose biological age was greater than their chronological age, which we measured by combining four different epigenetic clocks. At the time, three or four years ago, we combined two first-generation and two second-generation clocks because we had no idea which was better. Of course, this was super risky. Now we know more about their accuracy, especially the second-generation clocks. But the idea was to only include individuals who might actually need a molecule that interferes with epigenetic changes.
For the first time, the primary outcome parameter in a trial like this was the change in those same epigenetic clocks. The secondary outcomes were all the conventional clinical parameters we already know.
I think that’s the way we should go. We have the safety and recognition from the broader field of trialists, showing that we know how to randomize, we use the right software, and we apply tests properly. Then, as the cherry on top, we add all our other biomarkers to get a better understanding of how these interventions act. In other trials, we might use immune parameters or HbA1c as the primary outcome, and then new biomarkers of aging – based on the microbiome or epigenetics – as secondary outcomes.
Let’s talk about one of your most interesting trials, PROMETHEUS, which reached the semi-finals in the XPRIZE Healthspan competition. The idea is to build personalized regimens. What kind of signal can you get from regimens that are different for each person, and what type of functional decline are you trying to reverse, given that recovery of function is the XPRIZE criterion?
We are using the XPRIZE outcomes, which focus on immune, cognitive, and musculoskeletal function. We are measuring everything they require: CD4/CD8 ratio, muscle mass, muscle strength, and brain function using the NIH toolbox. Alongside that, we are including many other parameters like proteomics, epigenetics, microbiome data, and digital biomarkers to track changes in health.
The PROMETHEUS intervention is based on “gerotypes.” We only include individuals who are between the 25th and 75th percentile for these three functions. We exclude those who are doing very poorly and those who are doing very well. We are including the average person, like you and me.
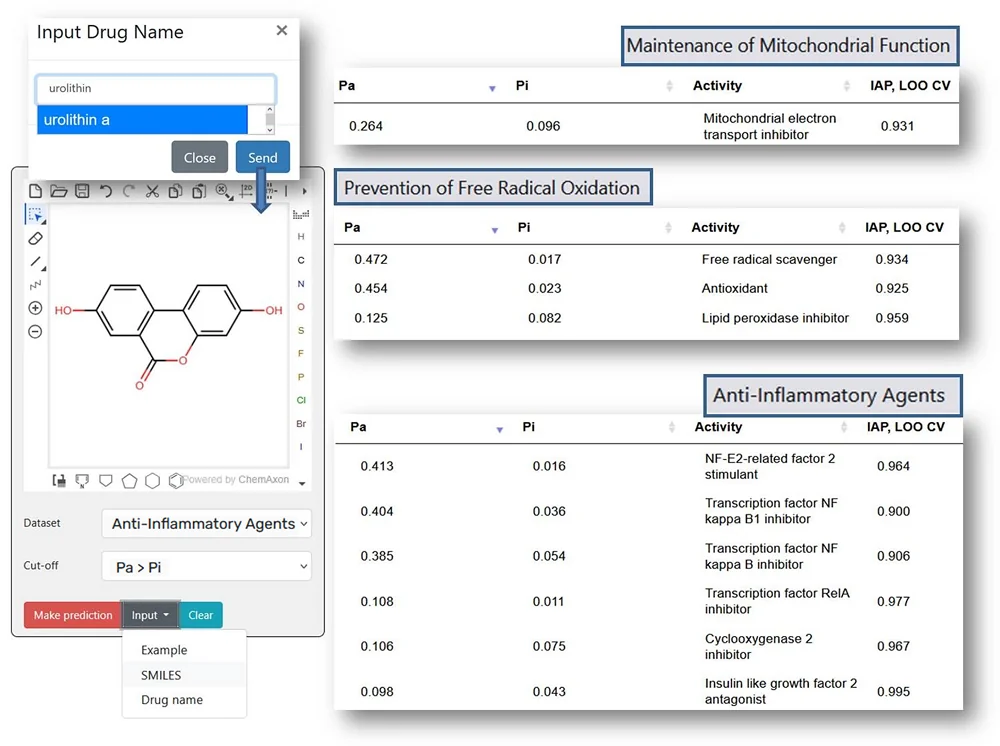
Based on these three areas – cognition, immune function, and muscle – we build a personalized regimen that includes lifestyle interventions, dietary recommendations, nutraceuticals, and, in the next stage, drugs targeting the individual’s specific gerotype. For example, if someone is low in muscle strength and mass, we would add urolithin A to their regimen, in addition to protein and creatine. We wouldn’t do that for someone who doesn’t have that specific weakness.
We built grids and flowcharts to standardize this personalized approach. It’s a huge list of nutraceuticals, including urolithin A, NMN, and ergothioneine – compounds where we have at least some human evidence of a signal and a very low likelihood of harm.
It sounds like a proof of concept for longevity medicine as a field, where you’re throwing all your knowledge at several cases to see what the best result you can get is.
Exactly. When we finish this trial, which is running now and is very intense – it’s like a boot camp for the participants – we won’t be able to disentangle exactly what worked for any single person. But we will know which combinations we prescribed based on their gerotype. Then, in the post-hoc analysis, we can see what was most likely to help.
But most importantly, we also measure compliance. We have a psychologist in the group who uses nudging techniques and helps us understand what people want to achieve. We are combining diagnostics from psychology to biology to the social environment to give our intervention the highest chance of working.
If it doesn’t work, we have to discover why. It could be because of the multimodal design, or because we don’t know if the effects are additive or if they cancel each other out. However, this is how we think in clinical practice. This is the first time we are strategically bringing that mindset into a clinical trial design.
You have several other trials running with interesting molecules such as AKG, fucoidan, rapamycin, and NMN. Do you have any juicy preliminary results you can share?
Yes. The ABLE study, which I’ve presented at a few conferences, really shows that we can’t just put longevity supplements on a shelf and let people decide for themselves whether to buy them. We do a lot of responder/non-responder analysis, and I think that’s the key issue for our field. We shouldn’t underestimate how heterogeneous the aging process is. Some people might have an epigenetic reprogramming problem, others a DNA damage problem, or issues with proteostasis.
Our AKG trial shows that we find significant epigenetic changes towards a lower biological age in individuals who were already biologically older, just by giving them one gram of calcium AKG for six months. But we find the signal is especially strong in individuals who are more physically active. Here you see that the combination of lifestyle and a supplement is very important. We also see better results in certain demographics. And we only applied it to individuals who were already epigenetically older – why would you charge your phone if it’s already full?
It’s the same for the NMN study we did a couple of years ago. After doing all the post-hoc analysis, we showed that the only individuals with a moderate NAD+ level increase showed clinical benefits from taking the NMN.
For me, it doesn’t matter if a trial’s primary outcome is positive or negative. The most important outcome is that we learn from the data. We have many non-responders in these trials, but we also have an equal number of people who are huge responders. The challenge for us in the coming years is to understand this responsiveness—who are the responders? That will show us who should actually be taking certain interventions in clinical practice.
I think your collaboration with the Alliance of Patient Organizations is particularly interesting. I’ve always thought that geroscience should get more support from patient advocacy groups. After all, we’re all patients when it comes to aging. Is this connection finally happening?
It is. It’s super active right now, though it only started six months ago. I was always looking for patient alliances, but it was hard because they were very specific: autism, menopause, colon cancer, and so on.
I have now found the overarching alliance of all patient organizations [Alliance of Patients Organizations Singapore – APOS]. We are now looking forward to building registries, giving good information to individuals, and recruiting from their members. It’s a hugely active and proactive partnership. I am so happy that we finally have the consumer at the table, in addition to healthcare professionals, industry partners, governments, and policymakers.
As someone who’s spearheading the longevity medicine movement, where does it stand today? I think it’s in an interesting spot. We don’t have many proven interventions, yet longevity clinics are popping up everywhere. And you are trying to build public longevity medicine, which seems critically important.
Healthy longevity medicine should grow both publicly and privately. However, what we really need is a very strong academic basis for it. If I could dream – and it seems the dream is coming true – we will have an alliance of academic clinics working together to build standards. We can test what works and what doesn’t in a non-private environment, from implementation strategies to cost-effectiveness to running randomized controlled trials together.
The reason we need academic centers to stand up is that we currently have zero capacity to teach and train the healthcare professionals who should be in this field. How can we grow private clinics if we have no capacity in normal teaching hospitals for people to be trained? It’s crazy.
We need to do the studies, but we also need teaching, teaching, and more teaching. Every medical specialty grew out of normal teaching capacities. If you go to a surgeon, you want that surgeon to be trained to do the job. Right now, we are just exposing ourselves to physicians who are not properly trained. I can’t exclude myself – I think I know what I’m doing, but I can’t prove that I’m a good physician in this field because there’s no one to certify me.
That’s what we have to solve now. Otherwise, this can become a very dangerous field. There’s a lot of ‘snake oil’ out there. We must establish a rigorous academic basis and have teaching hospitals that can offer rotations of six months, twelve months, or two years. We would never accept this lack of education and certification for oncologists. We would never accept it for surgeons. But we are accepting it at this moment for anyone who calls themselves a “longevity physician.” We have to do better.
We would like to ask you a small favor. We are a non-profit foundation, and unlike some other organizations, we have no shareholders and no products to sell you. All our news and educational content is free for everyone to read, but it does mean that we rely on the help of people like you. Every contribution, no matter if it’s big or small, supports independent journalism and sustains our future.