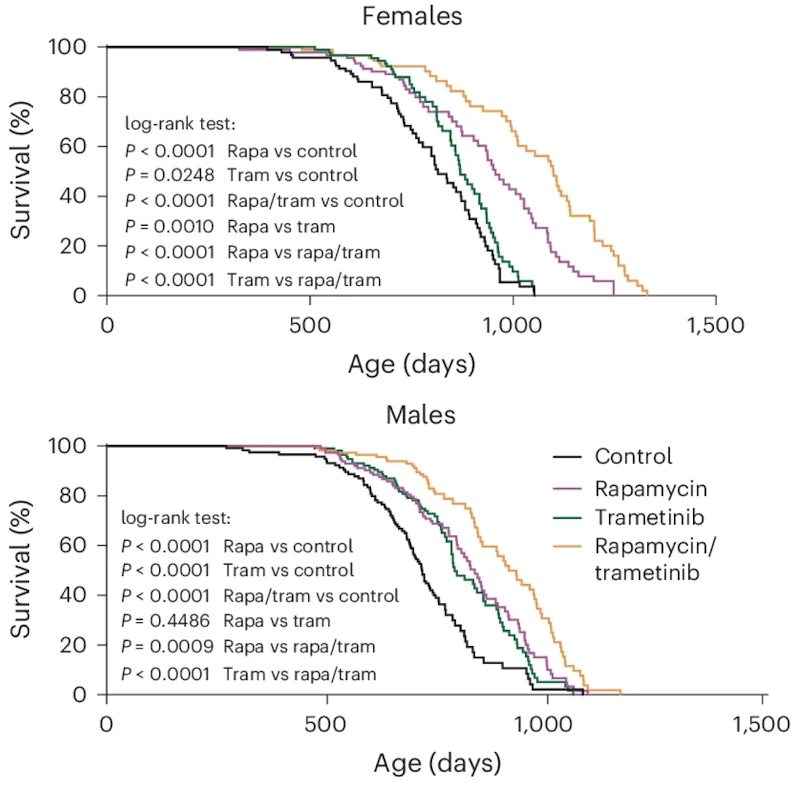
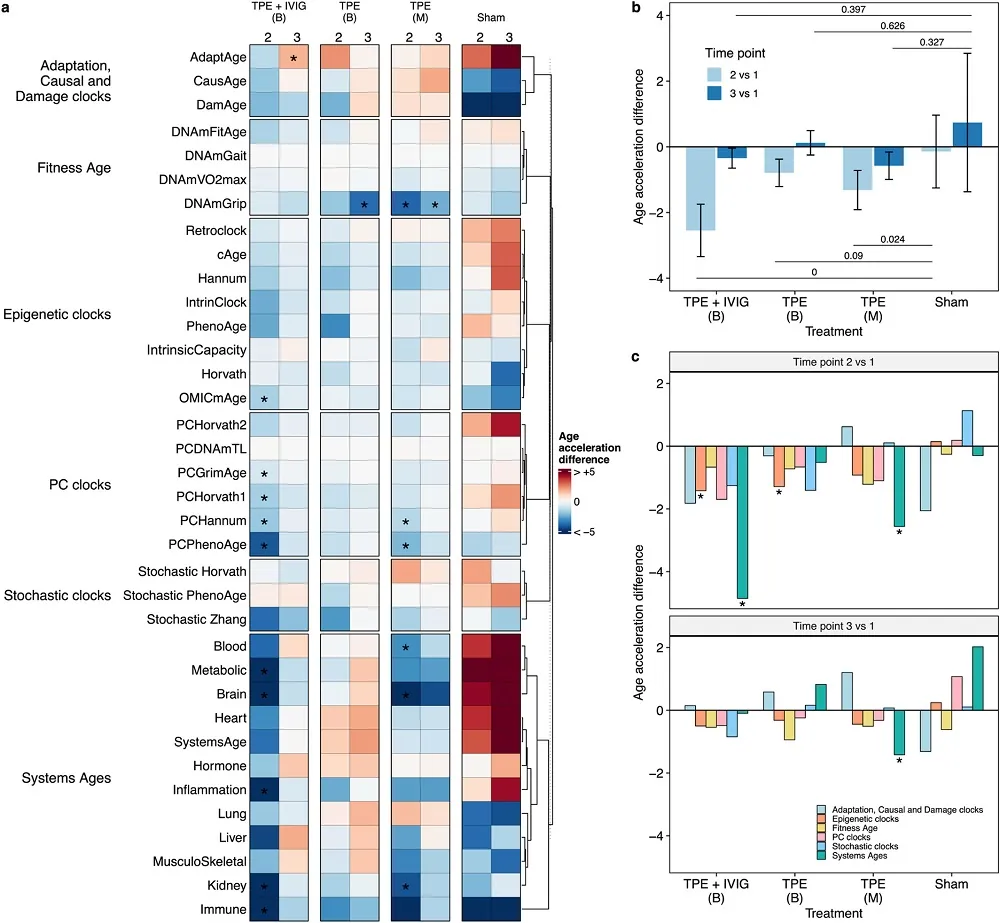
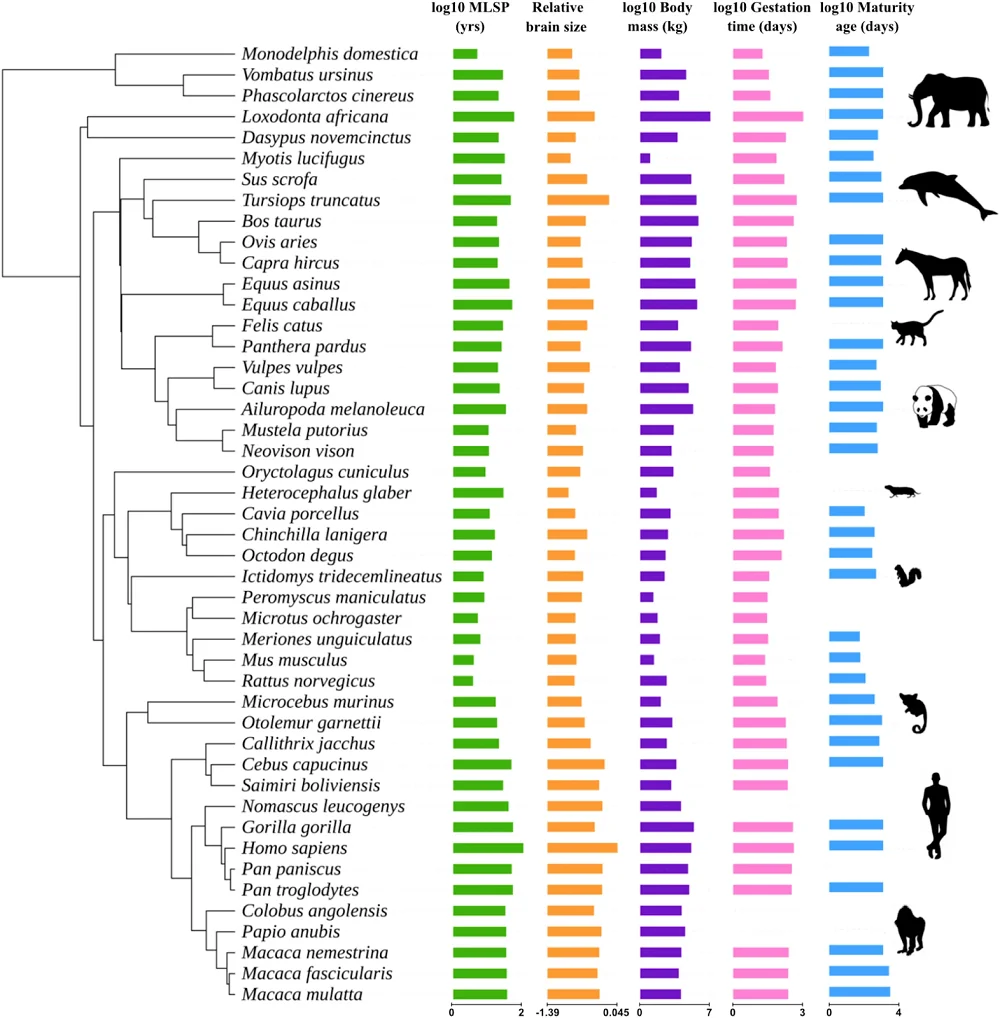
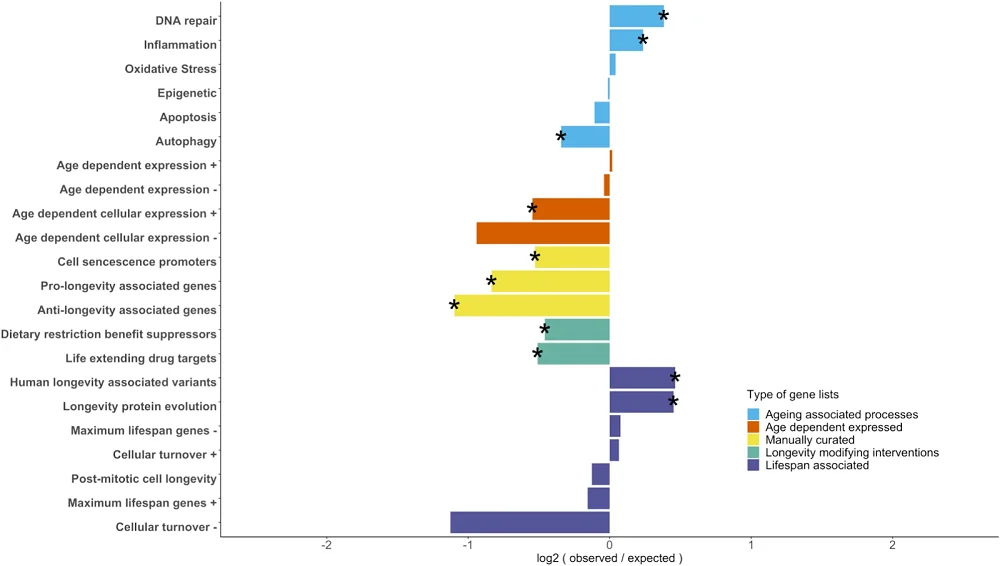
On its website, Rejuve.AI, a company co-founded by its dynamic CEO, Jasmine Smith, and a renowned AI researcher, Ben Goertzel, promises a lot of things: to “democratize longevity, globally,” to enable you to “take control of your data, and harness its earning potential,” and to “unite against aging.” We have been following the company for a while, and now that its app has been made available, we thought it was a good moment to sit with Jasmine and Ben for a talk and try to understand what’s behind these bold claims. Spoiler alert: Ben thinks that we’ll probably defeat aging quite soon.
So, Rejuve.AI just launched its app. However, it’s touted as something bigger than just a health app, as a longevity research network. Please explain that to me.
Ben: We want to help people in the immediate term by giving them tools to track their own lives and health better, but there’s a limit to what you can do that way. We’re interested in going even further. We want to encourage people to opt in to contribute the data from the app to a decentralized research commons. With luck, we can use our AI tools to discover new things about how to prolong healthy life and then roll back those discoveries to our first experimental test subjects, the app users, even before they’re rolled out to the public at large.
I think it’s a bit of a chicken-and-egg situation with human data in longevity research and biology in general. Data is the key element. You want to entice people to give you their health data and then use it. How?
Jasmine: The data is being used firstly to help the user improve their own health, but then to contribute to a wider body of knowledge, particularly internationally. We’re building a dataset that’s aiming to be better than NHANES, UK Biobank, and other regionally focused ones because it’s international and hopefully including more women and different minority groups.
We’re also tracking people’s personal self-experimentation journeys. Maybe somebody has something they swear by that really works for them: a certain diet, a supplement stack. Instead of being dismissive and saying, “That’s just an n=1” or “You can’t prove that,” we can actually see what types of people it works for and what the underlying biological mechanisms are. We can aggregate and expand those insights to help people save time and money on things that aren’t going to work.
Users can earn rewards in that same ecosystem, being incentivized to provide data accurately and on a regular basis. They can also be connected to trusted providers of services and consultations with clinicians to really implement these further and take it to the next level.
Ben: We don’t know how much data we actually need to fuel which AI discoveries about longevity. One focus of my own AI R&D has been to find approaches that can do more with less data, which is very critical in the biology domain. The successes we’ve seen with transformer neural nets and LLMs have been predicated on having massive amounts of data. That’s great when you have many variations of essentially the same English sentence, so an LLM can get a sense of its meaning from a large corpus.
With biology data, we’re in the opposite situation. There’s tremendous complexity within each human body and among human bodies, and the samples that we have, even if the world opened up all its medical data for open research, which is not the case, the samples would still be incredibly small compared to the depth and variety of the systems involved.
A lot of my work with the Rejuve.AI team has been on how to go beyond standard neural net methods. We’re looking at ensembles of neural nets helping each other learn, at neural-symbolic or evolutionary methods, even quantum computing approaches. How do you use more advanced techniques to make interesting imaginative leaps beyond the relatively small amounts of data to make the right generalizations about human longevity?
We need to be working from both directions. We need to make modern AI’s data requirements fewer, given the realities of gathering data from human bodies and their diversity. But we also need to increase the breadth and depth of available data and try to get the two to meet in the middle.
Like you said, even the types of data in biology are very diverse, so what are we after?
The nature of the data is as important as the amount. When you look at the research Big Pharma does, it’s more for economic reasons than anything else. They want to pick one disease, ideally one target, and pile resources on that one thing. This has sometimes yielded amazing results, such as statins, which are great for heart disease.
On the other hand, we all know aging is a holistic phenomenon. It’s about many different body systems, many different levels of the body working together. You do need drill-down data on very specific disease pathways, but you also need holistic data about what human bodies are doing during their lives, their overall health condition, diet, exercise, mood, and state of mind.
The mainstream biomedical world hasn’t been great at holistic approaches. Part of what we’re doing with the app is aiming to accumulate, over several years, the world’s largest corpus of holistic data about human bodies across their whole lifespan, both very healthy ones and less healthy ones.
We want both genomic data and clinical medical data, and lifestyle and psychological data; health knows no boundaries. By getting all this data conglomerated together and feeding it into machine learning systems, machine reasoning systems, proto-AGI systems, and then AGI systems, we hope that AI can make creative leaps beyond this data and generate interesting new hypotheses for prolonging human life.
The app we’ve launched now is a milestone because it’s the result of a lot of research. On the other hand, it’s certainly just step one. I live near Seattle, home of Amazon and Microsoft. So, to use an appropriate analogy, our app isn’t quite Windows 1.0, but maybe Windows 3.1; it’s decent, it does something, but there’s a long way to go.
I bet a lot of people in our age group can relate to this analogy. When you say, “it does something,” what exactly do you mean?
Ben: The app is very worthwhile now. I’m a user, we should all be users, but we’ll be adding more features step by step so that within a year, this will be a much more powerful tool. I think we could be 2-4 years away from a human-level AGI. The goal would be that once we get there, one of the first things this human-level AGI does is help us cure aging and death, and data like we’re gathering with the Rejuve app is going to help it do this trick.
Even if you have an AGI with slightly greater than human intelligence, it’s going to need data, and data takes time. Taking a therapy from an initial prototype through something considered safe enough for people to use takes time. Gathering data from a diversity of people over time also… takes time.
We want to ensure the AI has this data. I’m especially interested in a feature we’ll launch probably later this year or early next year, which lets app users launch their own clinical trials. You’d have groups of people trying something, maybe a paleo diet, maybe NAD supplements, and measuring the effects. These groups would agree to gather data about themselves in a certain way, creating a subset of more rigorously structured data that gives the AI more to work with.
The AI could also suggest clinical trials, saying “Do we have 200 people willing to try this supplement while not doing these other things and willing to provide this amount of data?” The AI itself can treat the world as its ethical opt-in laboratory and suggest trials.
It can generate hypotheses.
Ben: Right. Currently we’re using deep neural nets together with OpenCog Hyperon to generate hypotheses. We’re constrained by the slow pace of setting up collaborations with biology labs to test these hypotheses, but some experiments can be done by gathering data from app users and having people do standard lab tests rather than needing specialized biology lab equipment.
For hypotheses that can be tested “in the wild,” AI and people can brainstorm together, then groups can volunteer for trials. They’re doing good for the world and themselves. If therapies are discovered through a trial, participants could be early adopters when the therapy reaches the appropriate evaluation stage. These are features on our roadmap.
The current version of the app already provides significant value by structuring the process of capturing basic data about your life. You can get judgments on that data from the probabilistic AI model inside the app; that’s the basic platform we’re starting from. There are many other features coming: smarter AI, of course, but these flexible mechanisms for allowing the user community to self-organize and gather more targeted data are equally important as the AI advances.
This fits into the concept of citizen science, which is rapidly gaining traction, really well. It’s hard to do right, for instance, due to lack of randomization, but even though, the benefits can be huge.
Ben: It should get easier because even before we have AGI, we have AI now that can help with the science, which is interesting. As we integrate our OpenCog Hyperon with LLMs, we’ll get AI that’s better at experimental design, statistical data analysis, and hypothesis generation.
So much lab work can be outsourced now: you can send DNA, RNA, or metabolomic data and get a report back in your email. It is getting easier, but as you alluded to earlier, gathering appropriate data is still one of the weak points. That’s what makes biology such a challenge compared to AI work. With self-modifying AI, the AI is running and watching itself, and there’s the data. In biology, we’re dealing with all these biological bodies that aren’t properly instrumented for data collection.
There’s been a lot of activity lately in the field of experiment automation. Are you planning to test your hypotheses in animal models?
We’ve been working through our partner company, Rejuve Biotech, and SingularityNet, with a company based in Irvine, California that has fruit flies experimentally evolved over 45 years to live five to eight times longer.
The “Methuselah” flies.
Yes, but we have flies that are much better than the published Methuselah flies. We have a pipeline where we can take hypotheses from data analysis on the Rejuve network of human longevity enthusiasts and evaluate them on these long-lived fruit flies.
We can see whether feeding a combination of herbs or lifestyle modifications to the flies prolongs their life. Previously, we tried supplements in flies and then in people and found compounds that could roll back moderate or mild Alzheimer’s and decrease inflammation. You can also go the other way, using flies as a testbed for interventions discovered in humans.
As to automation, we can collect some data automatically from flies. You can put a camera above the cage and identify individual flies from their movement patterns, studying them using automated vision analysis, but I’ve been impressed by how manual some of the work still is. If you need a DNA sample from the nervous system of a fly, you’ve got a human looking through a microscope using tweezers. This seems very simple, but it’s a niche, so no one has bothered to automate it.
This relates to the acceleration we’ll get from even an early-stage AGI: not just amazing insights about aging but having an AGI that could synthesize new robots to wield the tweezers to extract the fly’s brain.
This would be groundbreaking.
Many things in biology could be sped up by a human-level AI operating robots and lab equipment. You should be able to speed up animal model experiments very rapidly and get much more accurate simulation models than we have now.
I’ve been working on this with Debbie, Rejuve.AI’s chief scientist, since around 2000. Right now, we could put together a thorough simulation model of the human body on different levels by combining all the simulation models from research literature with available datasets and using AI inference to fill the gaps. I think you could create a very nice holistic systems biology simulation of the human body at any point in time and as it ages.
No one seems to be doing that because systems biology isn’t in vogue. The pharma and academic worlds tend to micro-focus on particular subsystems of the body.
The data we’re gathering from the app will be critical because for a holistic simulation model of the human body, you need more holistic data about human bodies carrying out their lives alongside the micro-level data the biopharma industry provides. The data can help with specific therapeutics discovery and for configuring holistic systems biology simulation models of aging bodies. These simulation models, together with machine learning and reasoning, could drive significant discovery.
We have a partner project, NuNet, building decentralized computing infrastructure. In a future version of the app, users could donate processing power from their laptops, phones or company compute networks to run some of these simulations. App users would not only be contributing data but also idle processing cycles and memory, like a distributed longevity supercomputer.
Decentralized AI. Do you think it can work?
Ben: Absolutely. You can run evolutionary learning genetic algorithms for analyzing biomedical data, for example, very nicely in a widely decentralized way on heterogeneous compute infrastructure. One lesson from DeepSeek earlier this year is that you can scale down the processing power needed to train deep neural models. It’s becoming more evident that you can do a lot of AI work in a decentralized way, and even if some things still need server farms, you can do quite a lot of processing across a small number of edge devices. This is another way people can contribute.
We’re giving people multiple reasons to contribute. As we roll out future versions of the app, we’ll implement more sophisticated token incentivization mechanisms. You’re rewarded with RJV tokens for the data you provide, for insights toward discovery, and for contributing processing power.
It’s an interesting incentive mechanism. Can you tell me more about the idea of tokens and what you can buy with them?
Jasmine: We’ve spent a lot of time building essential partnerships to make this pipeline work. A few companies have been quick to adopt cryptocurrency; there’s this connection forming between crypto and longevity in this emerging DeSci space, which is exciting. We have GlycanAge, a peptide company, Travala for booking flights, hotels, and experiences with RJV tokens, a recent partnership for lab tests within the app, and a longevity and aesthetics clinic in Singapore onboarding RJV.
We’re seeing adoption, and we’re always trying to get our network partners to onboard so users can have that direct experience. The idea is that you earn tokens in exchange for providing data, then use them in a health and wellness ecosystem. We want to make the RJV token the currency for the longevity economy, for wearables, supplements, tests, consultations with clinicians, travel to therapies not available in your region, and so on.
We’re creating a circular economy, with tokens incentivizing more data provision to build up the database and eventually rewarding contributions to new products and therapies. That gets into our NFT program.
This sounds exciting, but when I downloaded and logged into the app, honestly, it felt a bit like a commercial gimmick. I wonder whether you’re concerned that you’ve created a too commercial image for the app, with all these collaborations pushing products and services.
Jasmine: These are all actual partners listed on our website too. We want a wide network with a variety of offerings, not favoring one over another. It’s about “Here are trusted sources if you’re looking for these things.” If you’re looking for NAD supplements, for example, this is a partner working with us in our network. We’re encouraging more partners to join and care about clinical validation, ethical standards, and certification of the products offered.
Ben: We’re trying to be careful, more so than many others in the longevity space. We’re only directly offering things we believe have actual value according to the science we know. There is a commercial aspect, but I take supplements and pay for them; that’s commercial reality. As we evolve the app, we’ll fine-tune the presentation.
My friend Bill who runs Life Extension Foundation faced a similar issue. He wants to help us all live forever, and they’re also selling supplements. He’s tried hard in Life Extension Magazine to present the scientific data behind each supplement. They won’t accept something for sale unless there’s peer-reviewed data showing it does what it claims. We’re aiming to position ourselves similarly: if someone wants to sell through us, it doesn’t mean we know it will definitely prolong life, but there’s scientific evidence making it a reasonable hypothesis.
Jasmine: And also a way for users to find and input data more easily, rather than wondering “Where do I go? Where do I get these tests?”
Your app offers an assessment of the user’s biological age, which means you’ve created a biological age clock. How was it done in terms of training and validation?
Jasmine: I wouldn’t call it a clock, more like a calculator at this point, though we’re continually enhancing it. We started with the NHANES dataset, the same one we’re using for our neural network model. We isolated about 150 variables that were closely correlated, using that as the ground state to gather more data and fine-tune. We want to augment it with DNA methylation data as well, since right now it’s based on blood biomarkers and survey metrics. We’re headed toward a multimodal approach.
The biological age feature itself is very attention-grabbing, and users love it. There was a debate because there’s not even a clinical standard for what biological age means or how to measure it. It’s more representative of whether you’re aging faster or slower compared to an average, and what you can do to improve. That’s where all biological age tests are right now.
I’ve been taking many of these tests myself recently, and the results are pretty congruent with what I could already imagine based on my lifestyle and metrics. Aggregating these numbers and seeing the different factors is valuable, but we want to move beyond just a number to something more informative.
Ben: It is statistically meaningful. Using these ML-based predictors, you can get a prediction that’s much better than noise about someone’s likely demise. It’s interesting to get this statistical indicator, but of course, there are error bars. It’s not like the Twilight Zone where someone could predict you’ll die on January 3, 2047. The risk is that people will over-interpret it, but we’ve tried to include safeguards in the app.
What will be most interesting is giving people the ability to inspect why the prediction was what it was, which relates to transparency in AI models. That’s limited in the current version, but we’re actively working on it. I’m 58; if I were told I’m biologically 75, I would certainly like to know why. The challenge, as with everything else, is data. We took data from the NHANES study.
I think NHANES is about 15 thousand people, but it’s a lot of data points because they tested many things.
Ben: The challenge is that most variables from that dataset aren’t available for the average app user. If someone had enough blood tests and all the information needed for the most informative NHANES-based model, you’d get a better statistical prediction. With the average person, you have only a few data points, resulting in an estimate with wider error bars due to lack of data.
The frustrating thing is that people often have a lot of data gathered about their bodies, but it’s sitting in databases of hospitals or clinical centers. Because we don’t own our own data, it’s easier to get all tests done again than to extract it from the databases of different medical facilities you’ve visited over your life.
This aspect of data sovereignty ties in with the blockchain underpinning. We talked about token incentivization and the potential use of decentralized processing power, but another interesting aspect of blockchain technology is that you own your data, have access to it, control who else can access it, and can trace it. This data can be stored in a decentralized cloud facility rather than wherever a doctor’s office or pharma company put it.
It’s insane that the medical world doesn’t work this way. Ideally, anytime you get a medical test, or a doctor writes a report on your condition, there would be a QR code you could scan to securely send that information to your account on a decentralized data repository. You could go online and see an index of all your uploaded data, encrypted by your private keys, and share whatever aspects you choose with whatever networks you want.
This would even simplify operations for the mainstream medical establishment. When you visit a new doctor, you’d just show your QR code, and they could scan whatever pieces of your data you thought appropriate to share. Currently, they have to go through all kinds of procedures to get information from out-of-network doctors. It would help the mainstream medical system work better while also giving projects like Rejuve tremendous amounts of data and enabling citizen science and decentralized science to work much better.
If we could just liberate the data, this in itself would be revolutionary. Ben, you’re a visionary. Do you have a timeline for how AI might help us solve aging?
Ben: I’m a fairly hardcore singularitarian. I think my friend Ray Kurzweil was probably roughly correct about AI reaching human-level intelligence around 2029. I’m hoping we can do it by 2027 or so, or it could even be next year.
But I think Ray was pessimistic about the timeline between human-level AGI and ASI. Once you have human-level AGI, it could be months to a few years before you have superintelligence. I don’t see why it would take 16 years as he thought, from 2029 to 2045, unless the AGI itself wanted to slow things down out of caution or because it liked Ray and wanted to make him correct. Looking at it that way, it’s unlikely we’ll come up with a definitive solution to human super-longevity before 2029-2031. We can make progress, but the major breakthroughs will likely come after that.
I’ll take that.
Ben: The question is what we can do now. You could say, “Just wait until AGI comes and let it solve longevity,” but an AGI isn’t magic. Just as humans are much smarter than most animals but aren’t magic—we still get sick and die, and I’d still lose a one-on-one fight with a tiger without weapons—an AGI will still have limitations.
The more data we can gather and intelligently organize to feed the AGI when it emerges, the faster it can progress on solving human aging. Even if an AGI gives us some magic pill, we’ll still want safety testing, trying it on small groups first, and so on.
By and large, if our singularitarian view is right, we’ll probably all be approximately immortal, barring freak accidents, by around 2035. If you get AGI in 2029, some progress toward ASI, and then five years for the AGI to solve things and create gene therapy clinics, that’s the optimistic timeline.
If this is overly optimistic and we don’t get human-level AGI until, say, 2040, which isn’t how I think things will go, but it’s not irrational, perhaps because it turns out human-level AGI needs quantum computing (which I don’t think it does) or because the global sociopolitical system goes even crazier in the wrong sort of way, then the scenario changes. If AGI arrives in 2040, then proto-AGI systems might make huge progress toward solving aging based on data from Rejuve and other sources before we reach human-level intelligence.
Assuming our own lives are important to us, we can hedge our bets. The same data is useful in either case. We can gather data, build longevity-based AI models, try out therapies, and if we get superintelligent AGI faster, all the better. If AGI development stalls, our proto-AGI may still make major longevity breakthroughs.
One conclusion I’ve reached after being in the future technology space for a while is that the problem isn’t whether to allocate resources to AGI versus longevity versus nanotech versus uplifting human consciousness. The problem is the total amount of resources spent on all this important stuff versus resources spent on destruction and selling useless junk. We need more AGI research, more super-longevity research, and to advance our longevity app. The more we can focus people’s energy on these things rather than on useless and destructive endeavors, the better off we’ll be.
We would like to ask you a small favor. We are a non-profit foundation, and unlike some other organizations, we have no shareholders and no products to sell you. All our news and educational content is free for everyone to read, but it does mean that we rely on the help of people like you. Every contribution, no matter if it’s big or small, supports independent journalism and sustains our future.



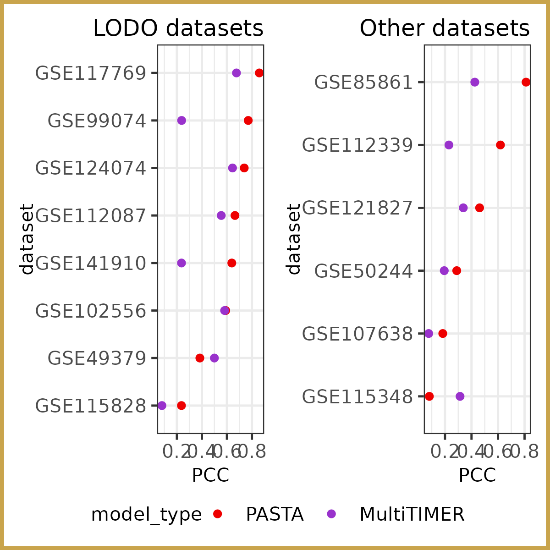
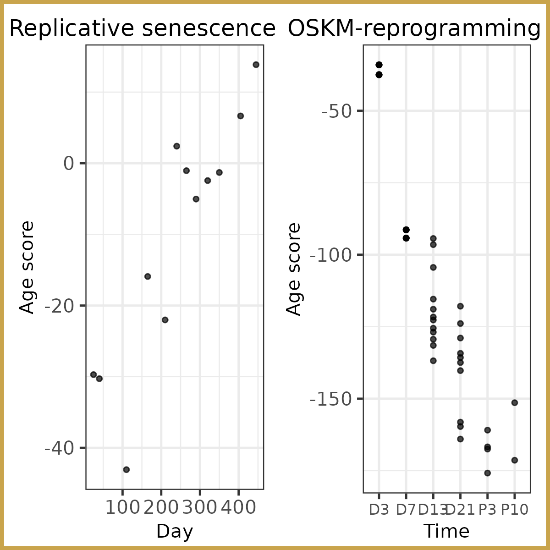
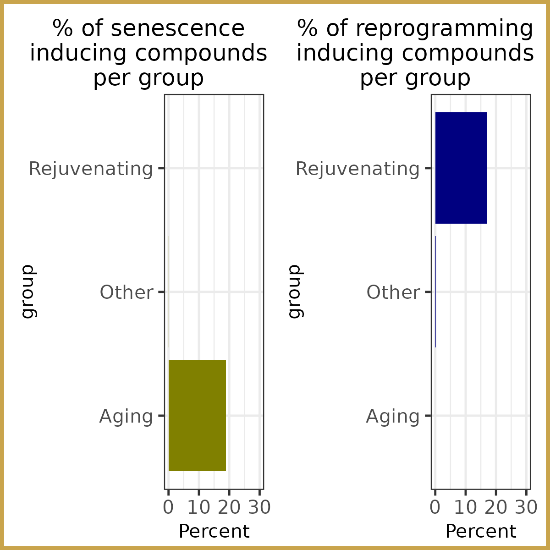


 Researchers at the Lifespan Research Institute have identified a
Researchers at the Lifespan Research Institute have identified a  Dr. Sajid Zalzala and his team have published the results of the Participatory Evaluation of Aging with Rapamycin for Longevity (
Dr. Sajid Zalzala and his team have published the results of the Participatory Evaluation of Aging with Rapamycin for Longevity ( Jay Olshansky kindly wrote a thought provoking op-ed for us back in January. His position is that
Jay Olshansky kindly wrote a thought provoking op-ed for us back in January. His position is that  Arkadi Mazin brought us this interesting
Arkadi Mazin brought us this interesting  Speaking of addressing the underlying biology of aging, Peter Fedichev appears to agree with Jay Olshansky.
Speaking of addressing the underlying biology of aging, Peter Fedichev appears to agree with Jay Olshansky.