How Life Expectancy Has Changed in Europe
A recent country-level analysis of life expectancy among several European nations shows changes in life expectancy trends and how well-designed national policies can reduce or minimize exposure to risk factors, thus improving life expectancy [1].
Slowdown in life expectancy increase
Life expectancy has grown in high-income countries since at least 1900, except during the two World Wars and the 1918 influenza pandemic [2]. However, the speed of the growth differed; for example, since 2011, Europe’s trend towards life expectancy increases was reduced, and this was followed by a decline in life expectancy in most European countries due to the COVID-19 pandemic [3, 4].
The authors of this recent study used the data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2021 to compare changes in life expectancy and exposure to risk factors in the 16 founding European Economic Area countries and four UK nations.
The life expectancy at birth was defined as “the mean number of years that a newborn infant could expect to live, if he or she were to pass through life exposed to the sex-specific and age-specific death rates prevailing at the time of his or her birth, in a given country.”
They compared periods of 1990 to 2011 (pre-slowdown in life expectancy), 2011 to 2019 (slowdown in life expectancy to pre-COVID-19 pandemic), and 2019 to 2021 (COVID-19 pandemic).
Country-level analysis
When analyzed separately, all countries showed improved life expectancy from 1990 to 2011 and 2011 to 2019; however, the rate varied. Confirming the observations of bulk data analysis reported previously, the rate of life expectancy improvement was higher in the 1990-to-2011 period than the 2011-to-2019 period.
Norway was the only exception from that observation. In Norway, the trend of life expectancy increased more during the 2011-to-2019 period compared to the 1990-to-2011 period.
During the COVID-19 pandemic and post-pandemic period, all countries but Ireland, Iceland, Sweden, Norway, Denmark, and Belgium experienced an absolute fall in life expectancy, with Greece and England observing the most significant decrease.


Cardiovascular diseases, cancers, and COVID-19
The life expectancy improvements seen in the 1990-to-2011 period stem from improvements related to causes of death attributed to cardiovascular diseases and neoplasms, which are tissue masses that result from abnormal growth, whether benign or cancerous.
Unsurprisingly, the decrease in life expectancy in years 2019–21 can be attributed to the deaths from respiratory infections and other COVID-19-related health problems. However, before the COVID-19 pandemic period, reductions in improvements in life expectancy were primarily driven by cardiovascular diseases.
The researchers also made an interesting observation: “among the studied countries, those with the greatest slowdown in life expectancy improvements before the COVID-19 pandemic were generally most severely affected by COVID-19 and had some of the largest decreases in life expectancy in 2019-21.”
Avoiding risk
The researchers analyzed risk factors, attributed to different causes of death, for both sexes in all countries combined in 2019. The top three risk factors for cardiovascular disease were high systolic blood pressure, dietary risks, and high LDL cholesterol. For neoplasms, the top risk factors included tobacco smoke, dietary risks, and occupational risks.
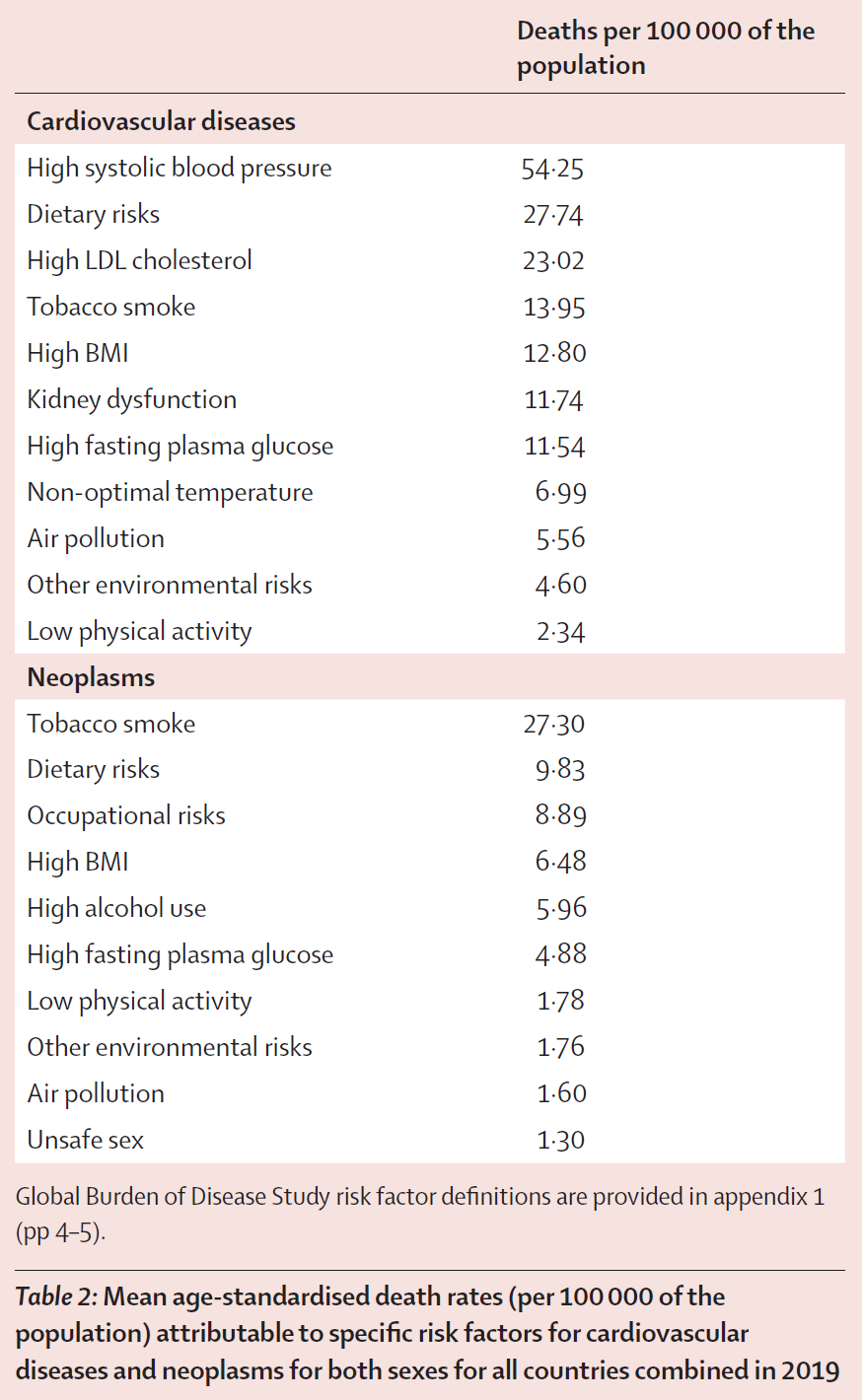
The levels of different risk factors changed with time, such as exposure to tobacco smoke; even though it is still a high population risk, it has decreased over time. On the other hand, BMI has increased in all countries, and dietary risks, high alcohol use, and low physical activity remain high in most.
The authors also point to high LDL cholesterol and systolic blood pressure, which declined until before 2011; however, this trend reversed after 2011 in many countries.
The danger of a risk factor varies by the time between exposure to it and the start of the disease that it causes, the length of exposure, and its interactions with other risk factors. Unfortunately, this dataset doesn’t provide some of that information.
Funding healthcare
Following their analysis, the authors discuss governmental policies and their impact on life expectancy. For example, they mention national fiscal and healthcare policies that impact the population’s life expectancy, especially for people in the worst socioeconomic situations.
An example of policies aimed at increase access to healthcare are Belgian, French, and Norwegian national policies, which, in recent years, have focused on increasing cancer diagnosis and treatment. The authors hold that these policies improved life expectancy related to neoplasms between 1990 and 2019. Additionally, some research has suggested that funding cuts to health, social care, and welfare since 2010 contributed to the slowdown in life expectancy improvement [5, 6].
Diet and physical activity are the foundation of health and longevity
Diagnosis and treatment happen after a person suffers from a disease. Preventing diseases from occurring through proper diet and physical activity might be more effective at increasing life expectancy.
The authors give examples of how healthy food consumption can be influenced by effective policy. An example is Norway, which had implemented a sugar tax as early as 1922. Similarly, starting in the 1980s, the Norwegian government talked with the industry about reducing the amount of salt in food products. This was complemented by Norway’s ‘Action Plan on Nutrition 2007–2011’, which, apart from education, also focused on other nutritional aspects, such as increasing focus on nutrition in a health care setting.
This broader approach has proven more effective than focusing only on education and voluntary dietary changes. Apart from diet, physical activity is the cornerstone of health and reducing premature mortality. Unfortunately, accordingly to this analysis, at the population level, there were no improvements in the levels of even low physical activity across the studied countries. The authors believe that systematic strategies and incentives are necessary to change that.
Ultimately, the authors intend for policymakers to utilize this analysis as a guide to reverse their countries’ slowdown in life expectancy improvement. They also hold that countries that implement successful policies can be used as examples for others to follow.
Literature
[1] GBD 2021 Europe Life Expectancy Collaborators (2025). Changing life expectancy in European countries 1990-2021: a subanalysis of causes and risk factors from the Global Burden of Disease Study 2021. The Lancet. Public health, 10(3), e172–e188.
[2] Roser M. (2020) The Spanish flu: the global impact of the largest influenza pandemic in history. https://ourworldindata.org/spanish-flu-largest-influenza-pandemic-in-history
[3] Raleigh VS. (2019) Trends in life expectancy in EU and other OECD countries. OECD Health Working Papers 108.
[4] Organisation for Economic Co-operation and Development, EU. (2018) Health at a glance: Europe 2018: state of health in the EU Cycle.
[5] Alexiou, A., Fahy, K., Mason, K., Bennett, D., Brown, H., Bambra, C., Taylor-Robinson, D., & Barr, B. (2021). Local government funding and life expectancy in England: a longitudinal ecological study. The Lancet. Public health, 6(9), e641–e647.
[6] McCartney, G., McMaster, R., Popham, F., Dundas, R., & Walsh, D. (2022). Is austerity a cause of slower improvements in mortality in high-income countries? A panel analysis. Social science & medicine (1982), 313, 115397.