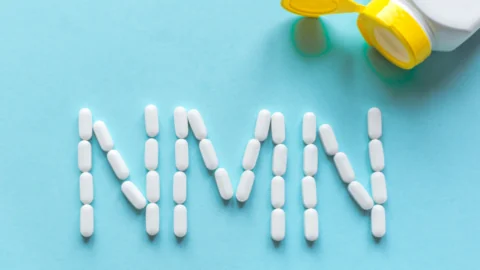
November 16, 2022
Reversing its own earlier decision, the FDA has informed an NMN manufacturer that the molecule can no longer be marketed as part of dietary supplements. Another company that is testing NMN as a drug probably contributed to the decision, which is not being enforced as of now. NAD and NMN NAD+ is a multi-role coenzyme...
November 04, 2019
A new publication by an international team of scientists has proposed a new healthcare framework to help older people stay healthier for longer by improving the development of therapies that target age-related diseases. Society is aging, and we need to change healthcare for the better This new publication urges World Health Organization (WHO), governments, and...
September 30, 2019
At our 2019 Ending Age-related Diseases conference in New York City, we had the pleasure of speaking with Dr. Michael West, the CEO of AgeX Therapeutics. Dr. West can rightfully be called a pioneer in his field with a substantial background in biomedical and biotechnology corporations. After completing his PhD at Baylor College of Medicine,...
July 19, 2018
A common concern in the community is that the FDA, the EMA, and other bodies, such as WHO, do not classify aging as a disease and that this poses a problem for developing therapies that target aging. However, this is not really as serious an issue as some people would suggest; today, we will have...