| A Groundbreaking Clinical Trial |
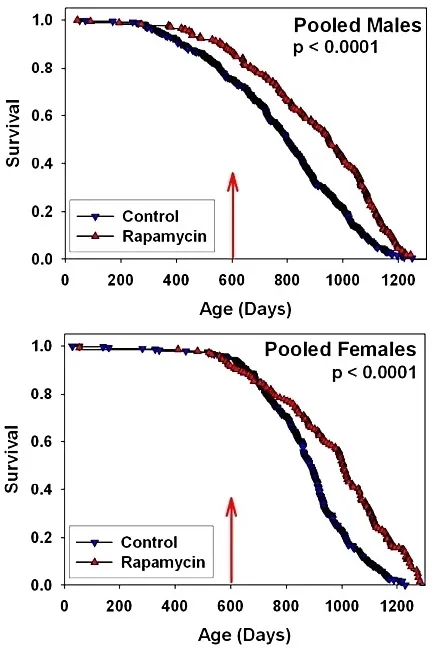
The medicine Rapamycin has been shown to extend the healthspan of all organisms it has been tested on – mice, worms, yeast – for decades, and yet to date there has been no trial to sufficiently demonstrate safety and proper dosing for this purpose in humans. It is now time for this to change.
With your help, we will be conducting a large clinical trial named Participatory Evaluation (of) Aging (with) Rapamycin (for) Longevity Study, or PEARL, to find out. This will be the first study to see if Rapamycin works as well in humans as it does in mice (for longevity).
Rapamycin was the first molecule proven to extend the lifespan of a mammal/mouse, even when given late in life (Harrison, D., Strong, R., Sharp, Z. et al. 2009). It also has the potential to treat aging related diseases (Blagosklonny, 2019) such as Alzheimer’s (Spilman P. et al. 2010) or heart disease, and not to mention, boost our immune system as well (Mannick JB. et al. 2014). It is by far the most studied longevity intervention after Calorie Restriction (CR).
Rapamycin works through the mTOR signaling pathway (Kennedy and Dudley 2016), one of the master regulators of cell metabolism and a key controller of autophagy (recycling in cells). Basically, it tells our cells to switch from growth to repair, and to clean out all the garbage. Not only that, but the quality of the proteins our cells produce increases, which means that there is less garbage in the first place. What all this amounts to is improved health and lengthened life for worms, flies and mice – now it’s our turn!
The PEARL trial will follow up to 200 participants over 12 months testing four different Rapamycin dosing regimens. It will be double-blind, randomized, placebo-controlled and registered with clinicaltrials.gov. The principal investigator is Dr. James P Watson at UCLA, who was also a PI for the famous TRIIM trial. To ensure safety the participants’ blood will be regularly monitored and side effects noted.
| How Will We Know If It’s Working? |
A battery of tests and measurements will be taken, both after 6 and 12 months. These will include autonomic health tests, blood tests, body composition tests, fecal microbiome testing, immune and inflammation health tests, methylation age clock testing and skeletal muscle tests.
With your help we will find out if and how well Rapamycin works to combat human aging. And, armed with a positive result, we will finally be able to help slow down onset of age related damage for you and those who you love and care about.

Interested patients will be screened for eligibility using AgelessRx‘s telemedicine platform. Eligible patients include those aged 50-85 of any sex or ethnicity, in relatively good health, and with only well-managed, clinically stable chronic diseases.
Once entered into the trial, the 200 patients will be divided into four dosing arms:
| 1. 2.5mg Rapamycin 3 times a week 2. 5mg Rapamycin once a week 3. 5mg Rapamycin twice a week 4. 10mg Rapamycin once a week |
And a placebo control arm – vital for both scientific credibility and statistical analysis. We have carefully selected these dosing regimens, allowing for recovery each week and with the total dose would not exceeding the regular dose of Rapamycin used in renal transplant patients (current FDA-approved regimen).
The safety of the participants is of paramount importance, so, apart from watching out for any side effects, their blood will be monitored by looking at CBC, electrolytes, lipids, liver and renal function.
The following tests will be conducted for evaluating efficacy:
| 1. Autonomic health tests – including baseline (am) heart rate and baseline (am) heart rate variability testing. 2. Blood tests – Standard measures of risk of age-associated diseases (glucoregulatory markers, lipids), and markers of inflammation. 3. Body composition testing with DXA scans – measuring bone density as well as visceral fat content. 4. Fecal Microbiome testing. 5. Immune Health Tests – including CD4/CD8 ratio, CMV IgG, TNFɑ, hsCRP, and IL-6. 6. Methylation age – clock testing (e.g. Horvath clock, GrimAge). 7. Skeletal muscle tests – including lean body mass on DXA scan. 8. Physiological data – wearable devices (steps, HRV, etc). |
The results of the trial will be published in an open access peer reviewed scientific journal.
In addition the data generated in the trial will be made available to any party.

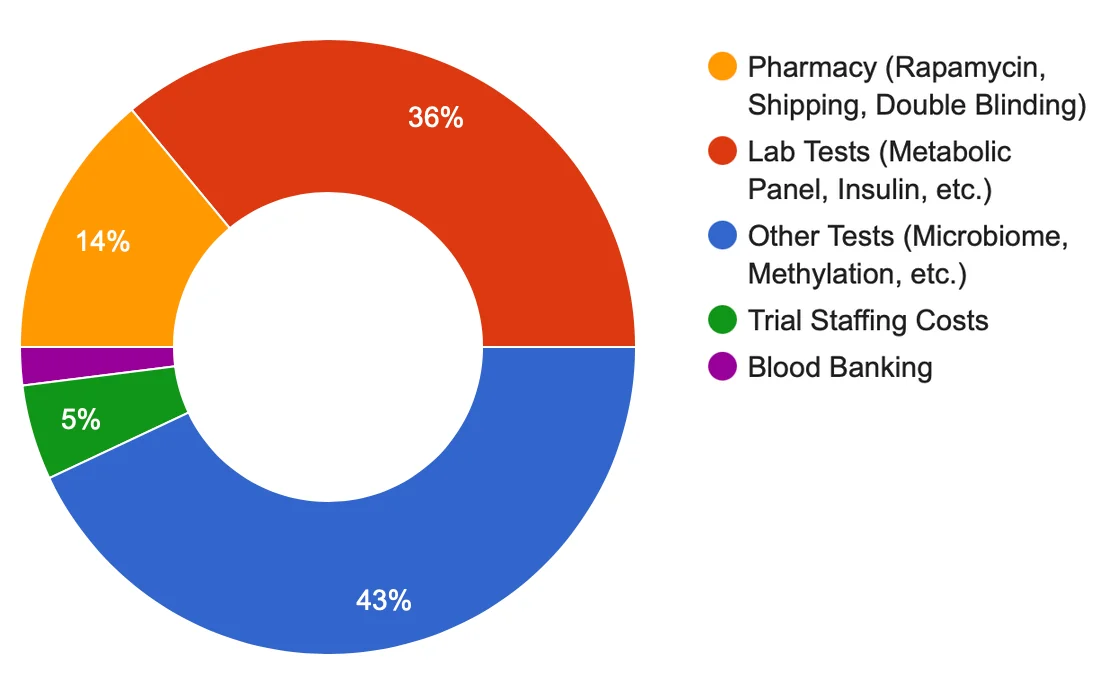 The most significant parts of our budget reflect the costs of performing of tests described in the PEARL protocol above — lab tests such as metabolic panels and insulin level analysis, and other tests such as microbiome and epigenetic clock biomarker testing — with the the remainder being allocated to sourcing the required amounts of Rapamycin and trial administration. While we’ve been able to mitigate costs thanks to the generous collaboration of Dr. Mikhail Blagosklonny at Roswell Park Comprehensive Cancer Center, James Clement at Better Humans, Adam Kadela at DexaFit, TruDiagnostic, GERO.ai, Mayo Clinic Translational Geroscience Network, Young.ai, and many others, it is still prohibitively expensive to run such a study with 200 participants. It is only thanks to the additional support of people like you that we will together be able to launch this trial, and answer key questions about Rapamycin’s efficacy to combat aging in humans. |

| $75,000 — Initial Goal Reaching our initial campaign goal will enable us to launch the PEARL trial to examine the healthspan extending effects of Rapamycin in humans. These funds, together with the generous support of our collaborators, will allow us to enroll 200 participants in this important study and run a battery of tests at the 6 month and 12 month mark: including autonomic health tests, blood tests, immune health tests, and skeletal muscle tests, along with more specialized testing at the 12 month mark: including microbiome testing, body composition testing via DXA scans, and methylation age testing with epigenetic markers such as the Horvath clock and GrimAge. Estimated study duration: ~12 months. |  | |||
| $120,000 — Stretch Goal 1 Attaining our first stretch goal will allow us to perform our entire battery of tests – including the specialized microbiome testing, body composition testing via DXA scans, and methylation age testing with epigenetic markers such as the Horvath clock and GrimAge — at the 6 month mark instead of only at the 12 month mark. Not only will this give us additional data points for longitudinal analysis, but will also be helpful in determining the minimal period of time Rapamycin dosing is required to see meaningful changes. All donors to this campaign at the $25 level or higher will be able to share in this process of discovery, seeing this data as soon as it becomes available. Estimated study duration: same as initial goal. |  | |||
| $165,000 — Stretch Goal 2 Reaching our final stretch goal will allow us to perform an entirely new set of proteomics testing at both the 6 month and 12 month mark. This is important because protein biomarkers can provide valuable insights into age related changes (Johnson A. et al. 2020) as well provide a comprehensive picture of chronic inflammation (Furman D. et al. 2019), a driving force behind age-related deterioration – “inflammaging”. It will also allow us to study the effect on proteins associated with immune system pathways, which is particularly important given Rapamycin’s known impact on immune system function. Including these tests within PEARL will therefore allow us collectively to gain the maximum amount of information we can regarding both the efficacy and safety of Rapamycin dosing regimens. Estimated study duration: same as initial goal. |  |
| What Happens Next? |
This first trial/phase is just the start.
It will be followed by a second trial/phase which will follow a larger number of participants over a longer period of time using the most effective dosing regime from the first trial/phase. It will also incorporate combinations with additional longevity drugs such as Metformin to assess any synergy with Rapamycin.
Furthermore, if this low cost and philanthropy funded trial succeeds, it will enable other longevity interventions to be tested – from generic drugs, to stem cell-based therapies, and beyond. Eventually, this will lead to the roll out of a wider variety of longevity interventions to the general public.
With your help, we can help transform the health of millions.
Thank you for your support!

Co-Founder and Chief Medical Officer of AgelessRx  Dr. Sajad Zalzala is the Co-Founder and Chief Medical Officer of AgelessRx. He combines the unique qualities of an experienced doctor passionate about disease prevention and longevity with the strategic vision and skills of a serial entrepreneur. Sajad has been passionate about slowing and reversing age-related diseases for 20 years. Sajad is one of the rare MD’s personally licensed in all 50 States, as well as Washington D.C. and Ontario. He has extensive experience working with startup companies in the telemedicine/telehealth field and has been an advisor to multiple successful healthcare startups, including Pill Club, Jack Health, forHims. Dr. Sajad Zalzala is the Co-Founder and Chief Medical Officer of AgelessRx. He combines the unique qualities of an experienced doctor passionate about disease prevention and longevity with the strategic vision and skills of a serial entrepreneur. Sajad has been passionate about slowing and reversing age-related diseases for 20 years. Sajad is one of the rare MD’s personally licensed in all 50 States, as well as Washington D.C. and Ontario. He has extensive experience working with startup companies in the telemedicine/telehealth field and has been an advisor to multiple successful healthcare startups, including Pill Club, Jack Health, forHims. | ||
Principal Investigator of PEARL Trial  Dr. Watson is a board-certified Physician and co-founder of University Stem Cell Center in Santa Monica, California, where he conducted two of the first IRB-approved Phase I/II clinical trials in the US of autologous fat-derived stem cells for cosmetic and reconstructive surgery. More recently, he has successfully completed two small clinical trials in anti-aging therapies, one utilizing Rapamycin and another utilizing a triple cocktail of drugs and supplements that were shown to reverse epigenetic age in immune cells. He co-founded a biotech company, Biostrux, designed to commercialize “point-of-care”, adipose-derived stem cells with a bioresorbable scaffold to do “in situ” tissue engineering with autologous stem cells, work for which he has obtained three grants from the DOD and NIH. He has authored or co-authored over 30 scientific articles and book chapters, and co-authors an online blog on aging called “AGINGSCIENCES™, Anti-Aging Firewalls™” that has 85,000 regular readers worldwide. He speaks English and Thai, having grown up in Bangkok and Phuket for the first 12 years of his life. Dr. Watson is a board-certified Physician and co-founder of University Stem Cell Center in Santa Monica, California, where he conducted two of the first IRB-approved Phase I/II clinical trials in the US of autologous fat-derived stem cells for cosmetic and reconstructive surgery. More recently, he has successfully completed two small clinical trials in anti-aging therapies, one utilizing Rapamycin and another utilizing a triple cocktail of drugs and supplements that were shown to reverse epigenetic age in immune cells. He co-founded a biotech company, Biostrux, designed to commercialize “point-of-care”, adipose-derived stem cells with a bioresorbable scaffold to do “in situ” tissue engineering with autologous stem cells, work for which he has obtained three grants from the DOD and NIH. He has authored or co-authored over 30 scientific articles and book chapters, and co-authors an online blog on aging called “AGINGSCIENCES™, Anti-Aging Firewalls™” that has 85,000 regular readers worldwide. He speaks English and Thai, having grown up in Bangkok and Phuket for the first 12 years of his life. | ||
Co-Founder and CEO of AgelessRX  Anar is Co-founder and CEO of AgelessRx. He is passionate about advancing the science of overcoming the negative effects of aging and giving everyone convenient and affordable access to the latest technologies. He has played an instrumental role in organizing the PEARL trial and establishing relationships with all collaborating partners. His hope is that this project will bring us one step closer to having a panel of “longevity” biomarkers as well as open the door for more citizen-based and philanthropy funded trials. Anar is Co-founder and CEO of AgelessRx. He is passionate about advancing the science of overcoming the negative effects of aging and giving everyone convenient and affordable access to the latest technologies. He has played an instrumental role in organizing the PEARL trial and establishing relationships with all collaborating partners. His hope is that this project will bring us one step closer to having a panel of “longevity” biomarkers as well as open the door for more citizen-based and philanthropy funded trials. |















| Contributions to this campaign are received as donations to LEAF via Fiscal Sponsorship. |
August 2024 Update - PEARL
We joined AgelessRx and Dr. Matt Kaeberlein to discuss the results of the PEARL clinical trial for Rapamycin.
January 2024 Update - PEARL
The PEARL team at AgelessRX has informed us the study has concluded:
We are excited to share with you that all study participants have successfully concluded the final dosage of the study medication. Currently, Dr. Zalzala and our clinical team are diligently analyzing the collected data. Our aim is to complete this comprehensive analysis in the coming months, and we commit to keeping you informed of any significant progress.
October 2023 Update - PEARL
August 2023 Update - PEARL
Good news! We have received an update from the PEARL team. Clinical trials are a slow and time consuming process, but there has been some progress which appears below:
We are grateful for your continued engagement and interest in the PEARL trial. We are excited to provide an update on our progress and the current status of the trial.
Trial Progress Highlights:
As of 08/29/23, we are pleased to report that the PEARL trial has successfully enrolled 122 individuals. Among them, 59 participants have successfully completed the trial, 53 participants are on track to conclude their participation by the end of December 2023, and 10 participants have withdrawn from the study.
Data Analysis and Insights:
Our dedicated clinical team has accumulated a variety of data from our participants over the past year. Such data includes DXA scans, Quest bloodwork panels, DNA Methylation, gut health, and more. The next phase, which we are now setting infrastructure up for, involves an in-depth analysis to gather meaningful insights.
Safety and Adverse Events Monitoring:
Regarding participant safety and engagement. Unfortunately, we lost one participant due to causes unrelated to the trial medication. The remaining 9 participants opted to withdraw from the trial due to side effects or their hesitance regarding the placebo arm.
Data Collection and Partnerships:
Our data collection process is proceeding as expected. Home-based kits from TruDiagnostics, Thorne, and GlycanAge continue to be a key part of our data collection strategy. Ongoing quality assurance checks have been conducted to validate the accuracy of submitted kits. On the in-person front, safety blood panels and DEXA scans are proceeding as expected. We’ve also managed to biobank blood/plasma/serum pre/post samples from 30 participants for future analysis.
Pharmacy Supply and Participant Support:
We are pleased to share that participants have been consistently receiving their medications (5mg, 10mg, & Placebo) from our partner pharmacy without any supply or shipping disruptions.
Upcoming Webinar and Future Milestones:
In September, we are excited to record and share an “Interpreting Your Results” webinar hosted by Dr. Zalzala. This session aims to empower participants by educating them on how to read their individual results from bloodwork, DEXA scans, at-home kits, and post-trial information.
October 2021 Update Update - PEARL
Quick PEARL Update
The Participatory Evaluation of Aging with Rapamycin for Longevity (PEARL) Trial has been a focal point for our research efforts. After successfully closing a crowdfunding campaign, raising over $180,000, we’re in the active recruiting and enrolling phase of the project. Our aim is to establish a long-term safety profile, determine the long-term efficacy of Rapamycin in reducing clinical aging measures, and biochemical and physiological endpoints associated with declining health and aging in healthy older adults.
The official PEARL protocol can be found on the U.S. National Library of Medicine’s Clinical Trials webpage here.
What’s next? We’re excited to announce that we’ve begun onboarding PEARL participants and will continue recruiting on a rolling basis. If you’re interested in learning more on how you may qualify for PEARL, check out eligibility requirements and additional information here.
October 2021 Update - Pride Pledges Now Shipping
Just a quick update to let you know that we are now shipping the pride packs with buttons and stickers to backers starting this week. We are also preparing the books and T-shirts to ship just as soon as the garments have been printed and returned to us.
July 2021 Update - Rewards
Thank you once gain for supporting the PEARL campaign!
This is just a quick update to let you know that we engaging with our reward fulfilment manager and will be arranging the production and shipping of the T-shirts, buttons, and other physical rewards in the coming weeks.
For the non-physical rewards such as Q&A with the team we will be following up with you soon. We will keep you updated as things develop.
Thank you, and all of you in the USA have a Happy 4th of July!