February 22, 2021
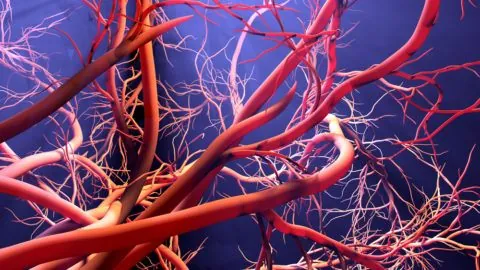
Tissue engineering company Humacyte is about to make a special purpose acquisition company (SPAC) deal in order to go public. The company is developing bioengineered Human Acellular Vessels (HAVs), which provide prosthetic vasculature to support the repair, reconstruction, and replacement of blood vessels damaged by injury or disease. What is a SPAC? A special purpose...
July 19, 2018
A common concern in the community is that the FDA, the EMA, and other bodies, such as WHO, do not classify aging as a disease and that this poses a problem for developing therapies that target aging. However, this is not really as serious an issue as some people would suggest; today, we will have...
February 20, 2018
Back in November 2017, the FDA announced a comprehensive policy framework for the development and oversight of regenerative medicine products, including novel cellular therapies. Both draft guidance documents had 90-day comment periods, and we at LEAF joined forces with the Niskanen Center to submit comments to the FDA to ensure that the voice of the...