Why Some Mammals Live Much Longer Than Others
- Maximum lifespan potential is associated with immune function.

- Copies of certain genes are strongly associated with greater lifespan.
- There are also associations between lifespan, brain size, and the immune system.
- This line of research may provide targets for lifespan interventions.
A recent study investigated differences in maximum lifespan potential among different mammalian species. The researchers found associations between gene family size expansion, maximum lifespan potential, and relative brain size. They also studied genomic features linked to lifespan evolution [1].
Maximum lifespan potential
Maximum lifespan potential can be defined as “the age at death (longevity) of the longest-lived individual ever recorded in a species,” both in the wild and in captivity, where such risks of death from predation or limited resources are not present.
Intrinsic biological factors determine maximum lifespan potential, and it varies widely among mammals, from less than a year for some of the shrew species to even two hundred in bowhead whales. These species’ genetic differences have been studied in order to examine the underlying biological processes that lead to such differences in lifespan. Previous work has identified changes in genes related to DNA repair, cell-cycle regulation, cancer, and aging in bowhead whales [2] along with expansion in gene families associated with DNA repair and tumour suppression in elephants [3]. This study of genetic differences and related molecular processes may be useful for the development of longevity interventions.
Some studies have explored how maximum lifespan potential is impacted by gene expression differences, gene family size, and similar genomic measures [4, 5]. These studies have pointed to the evolution of gene family size as an essential player in maximum lifespan potential.
Gene families are created when a single gene is duplicated. In such an event, the extra copy has more freedom to evolve, as the original copy produces the protein needed for the organism. The second copy can become a pseudogene, one that has accumulated so many mutations that it ceases to work correctly. Alternatively, it can mutate into a protein similar to the original but with a slightly different function, giving an organism a potential evolutionary advantage. This process can be repeated multiple times, creating a gene family of similar, but somewhat different, genes. Studies of bowhead whales and naked mole rats suggest that some of these duplications are linked to the increased longevity of those animals [2, 6].
In this study, the researchers built on those observations and compared the impact of gene family size on maximum lifespan potential in multiple mammalian species.
Brain size matters
The researchers conducted the bioinformatics analysis of 4,136 gene families in 46 fully sequenced mammalian species. They found an association between maximum lifespan potential and the expansion of 236 gene families.
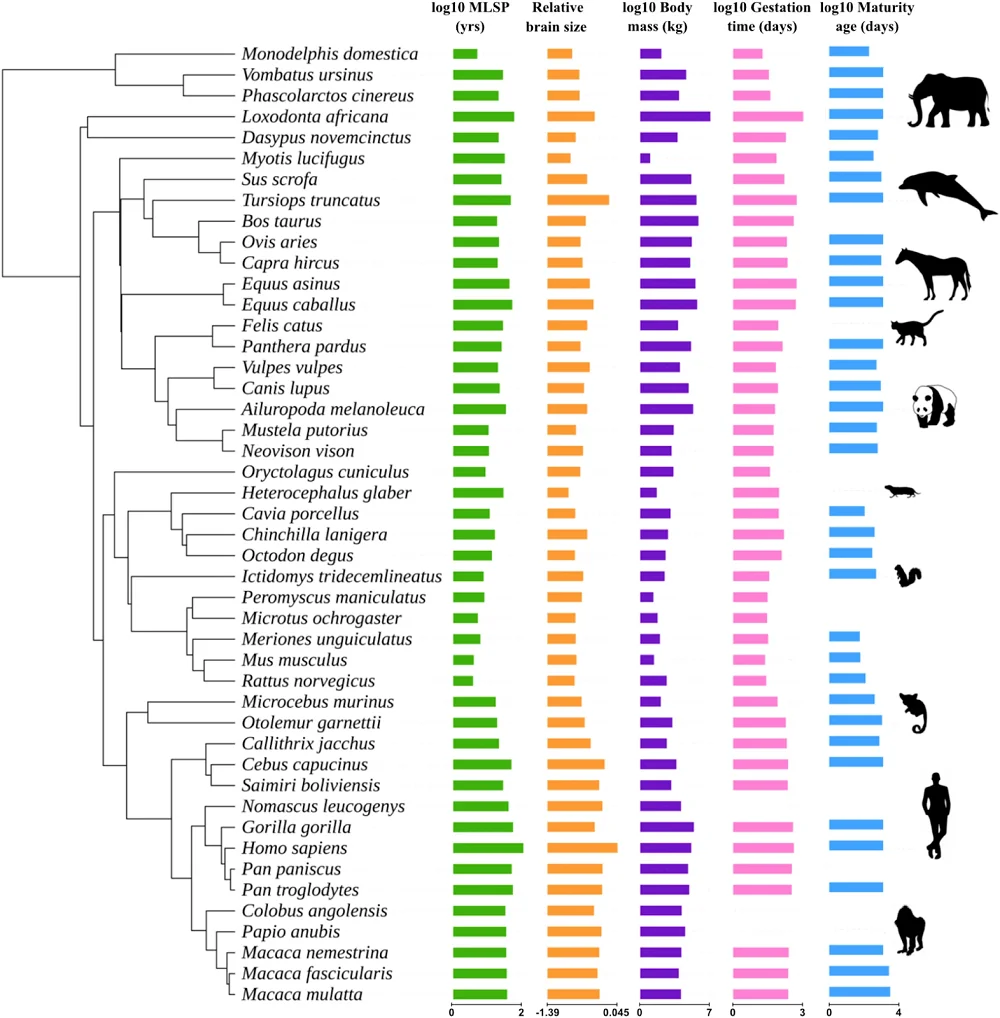
Next, they tested potential confounders, which can affect the results. They tested relative brain size, body mass, gestation time, and age at sexual maturity. Only relative brain size was found to influence the association of gene family expansion with maximum lifespan potential. These results are in line with previous research suggesting that the evolution of larger brains is related to maximum lifespan potential. The researchers also observed that gene groups related to maximum lifespan potential and gene groups related to brain size were also more likely to contain genes related to immune functions.
The researchers discuss that the immune system can positively impact a longer lifespan in multiple ways, such as through removing senescent cells, infectious agents, and potentially cancerous cells.
However, these results do not have a straightforward interpretation, as the researchers’ sensitivity analysis indicated that most species included in the study have a negligible effect on the results. Larger effects were observed for a few species, suggesting that while one species does not drive the results, they can be impacted by animal groups (taxa) that have extreme values.
More gene diversity
The researchers hypothesized that the expansion of gene families associated with the evolution of maximum lifespan potential might be related to the amount of gene product available in the cell (gene dosage) or the diversity of gene transcripts.
Transcript diversity is related to a process called alternative splicing. Mammalian genes are built from coding DNA sequences (exons) interspaced by non-coding DNA sequences (introns). When DNA is transcribed into RNA during protein production, introns are removed and exons are connected. However, exons are not always spliced in the same order, and, sometimes, some exons are skipped, creating alternative protein versions that originate from the same gene.
Comparing human maximum lifespan potential-associated genes with other background genes revealed higher gene expression levels and a higher number of unique transcripts among maximum lifespan potential-associated genes.
However, the authors warn that these results must also be interpreted with caution as they are only based on human data, and such observations might not be accurate for other species; future studies need to dive deeper into the evolutionary significance of this observation.
Functional, but not a single gene overlap
The researchers gathered data from previous studies that identified different aging-associated genes. They divided them into groups of genes related to aging-associated processes, genes whose expression is age-dependent, manually curated genes associated with ageing or longevity, targets of longevity-modifying interventions, and lifespan-associated genes.
Comparing age-related process genes with maximum lifespan potential-associated genes showed that the latter group is significantly enriched in genes related to DNA repair and inflammation; however, autophagy-associated genes were underrepresented.
Among the genes with age-dependent expression, researchers observed either underrepresentation among maximum lifespan potential-associated genes or didn’t find under- or over-representation, depending on the database and whether their activity increased or decreased with age.
The manually curated genes for cellular senescence and longevity, as well as genes that respond to longevity-modifying interventions such as caloric restriction and life-extending drugs, were significantly underrepresented among maximum lifespan potential-associated genes.
Only genes that have human centenarian-associated genetic variants and genes with faster protein evolution in species with higher maximum lifespan potential were over-represented among maximum lifespan potential-associated genes.
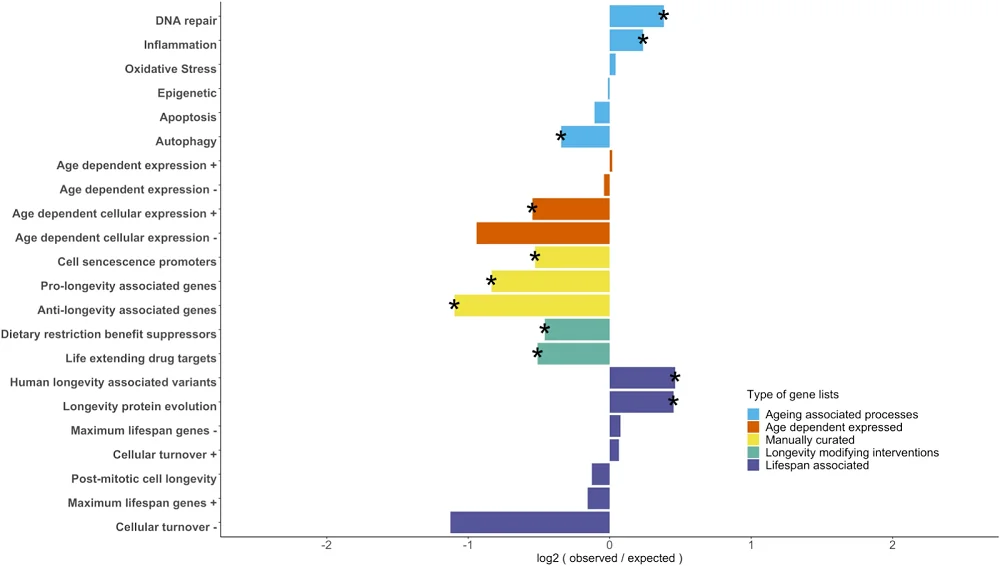
In general, there was a limited overlap between single gene lists from this and previous studies. However, there is an overlap regarding the functions and processes in which those genes are involved. The researchers identified this overlap in immune system functions, DNA damage and repair, apoptosis, autophagy, senescence, and life-extending drug targets, They conclude that “while different studies may identify distinct gene sets, they often highlight the same biological pathways, reinforcing the importance of these processes in longevity.”
While this study does not allow for establishing causality but only associations, its results help in understanding the evolutionary basis of a longer lifespan and identify the genetic and molecular processes that increase maximum lifespan potential.
Literature
[1] Kilili, H., Padilla-Morales, B., Castillo-Morales, A., Monzón-Sandoval, J., Díaz-Barba, K., Cornejo-Paramo, P., Vincze, O., Giraudeau, M., Bush, S. J., Li, Z., Chen, L., Mourkas, E., Ancona, S., Gonzalez-Voyer, A., Cortez, D., Gutierrez, H., Székely, T., Acuña-Alonzo, A. P., & Urrutia, A. O. (2025). Maximum lifespan and brain size in mammals are associated with gene family size expansion related to immune system functions. Scientific reports, 15(1), 15087.
[2] Keane, M., Semeiks, J., Webb, A. E., Li, Y. I., Quesada, V., Craig, T., Madsen, L. B., van Dam, S., Brawand, D., Marques, P. I., Michalak, P., Kang, L., Bhak, J., Yim, H. S., Grishin, N. V., Nielsen, N. H., Heide-Jørgensen, M. P., Oziolor, E. M., Matson, C. W., Church, G. M., … de Magalhães, J. P. (2015). Insights into the evolution of longevity from the bowhead whale genome. Cell reports, 10(1), 112–122.
[3] Chusyd, D. E., Ackermans, N. L., Austad, S. N., Hof, P. R., Mielke, M. M., Sherwood, C. C., & Allison, D. B. (2021). Aging: What We Can Learn From Elephants. Frontiers in aging, 2, 726714.
[4] de Magalhães, J. P., Curado, J., & Church, G. M. (2009). Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics (Oxford, England), 25(7), 875–881.
[5] Fushan, A. A., Turanov, A. A., Lee, S. G., Kim, E. B., Lobanov, A. V., Yim, S. H., Buffenstein, R., Lee, S. R., Chang, K. T., Rhee, H., Kim, J. S., Yang, K. S., & Gladyshev, V. N. (2015). Gene expression defines natural changes in mammalian lifespan. Aging cell, 14(3), 352–365.
[6] Kim, E. B., Fang, X., Fushan, A. A., Huang, Z., Lobanov, A. V., Han, L., Marino, S. M., Sun, X., Turanov, A. A., Yang, P., Yim, S. H., Zhao, X., Kasaikina, M. V., Stoletzki, N., Peng, C., Polak, P., Xiong, Z., Kiezun, A., Zhu, Y., Chen, Y., … Gladyshev, V. N. (2011). Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature, 479(7372), 223–227.







