Personalized Medicine Approach to Senolytics Clinical Trials
- Senolytics might work better in people with higher senescent cell burdens.

- Creating clinical trials that only target people with higher senescent cell burdens may lead to higher success rates.
- More potent and targeted senolytics may affect more people.
Recent commentary in Nature Aging summarized the results of clinical trials for senolytics and discussed recommendations for future clinical trials that use personalized medicine approaches [1].
From basic science to clinical trials

Read More
Destroying senescent cells using senolytics may mitigate some aging-associated symptoms or diseases. Preclinical studies on senolytics have determined that they can induce many beneficial effects, thus prompting researchers to test them against various conditions in human clinical trials. Those studies have determined that senolytics appear to be safe for use in people.
The authors of this commentary summarized the results of those clinical trials, dividing them into two groups. The first encompassed trials that used senolytics as systemic treatments, and the second encompassed trials in which senolytics were used locally due to possible toxicity. The discussion mainly focused on the systemic treatment trials.
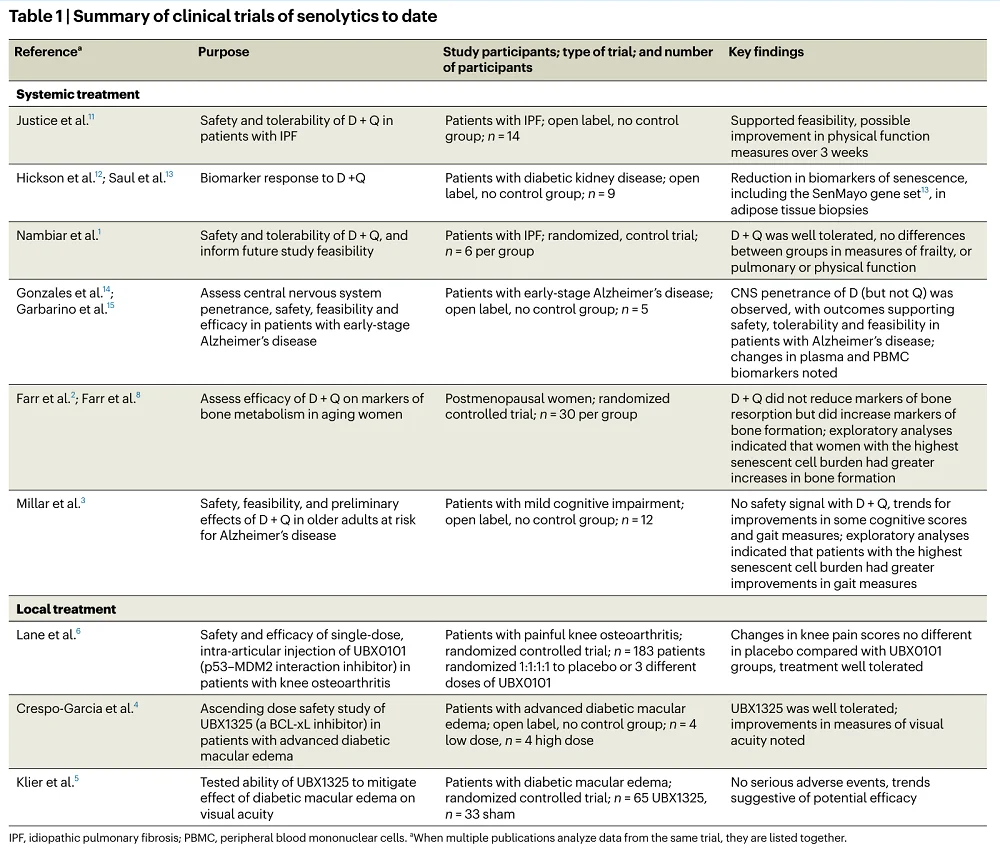
Of the systemic treatments, only the combination of dasatinib and quercetin has been tested; those trials included a small number of participants, with feasibility and safety being the primary focus. While some trials suggest efficacy, the authors caution against drawing firm conclusions since some studies didn’t have control groups to compare against.
Systemic senolytic treatment was also used in two randomized controlled trials. One of them was published by the same group that wrote this commentary, and it involved the most extensive study of this type with 60 postmenopausal women [2]. The researchers observed “a positive signal for an increase” in the bone formation marker procollagen type 1 N-propeptide (P1NP). They note that analyzing this study suggested some improvements that can be applied in the future design of senolytics trials. To explain this, they dived deeper into the results.
Assessing senescent cell burden
First, they describe that when analyzing the entire study population, they observed an increase in the bone formation marker P1NP, but it was modest (16% increase compared to controls). However, the study hypothesized that “the baseline senescent cell burden would determine the clinical response to the senolytic intervention.” To test that hypothesis, the researchers needed to assess senescence burden by measuring the gene expression of p16 in T cells. However, since such measurements are challenging, they also conducted additional tests and measured a panel of 36 SASP factors.
When women were divided into groups based on T cell expression of p16 mRNA, the women in the highest tertile (T3) had the highest P1NP levels. They also identified six SASP factors that showed increased levels at baseline in the T3 group compared to the T1 and T2 groups. They used those six factors to develop a SASP score that indicated senescent cell burden and used this score to predict responses to dasatinib and quercetin treatment: the SASP-score T3 group showed a similar increase in P1NP as the T cell p16 T3 group. Moreover, selecting participants in both the SASP-score T3 group and the T cell p16 T3 group (the people with the highest senescent cell burden) showed even higher levels of P1NP following dasatinib and quercetin treatment, supporting the original hypothesis.
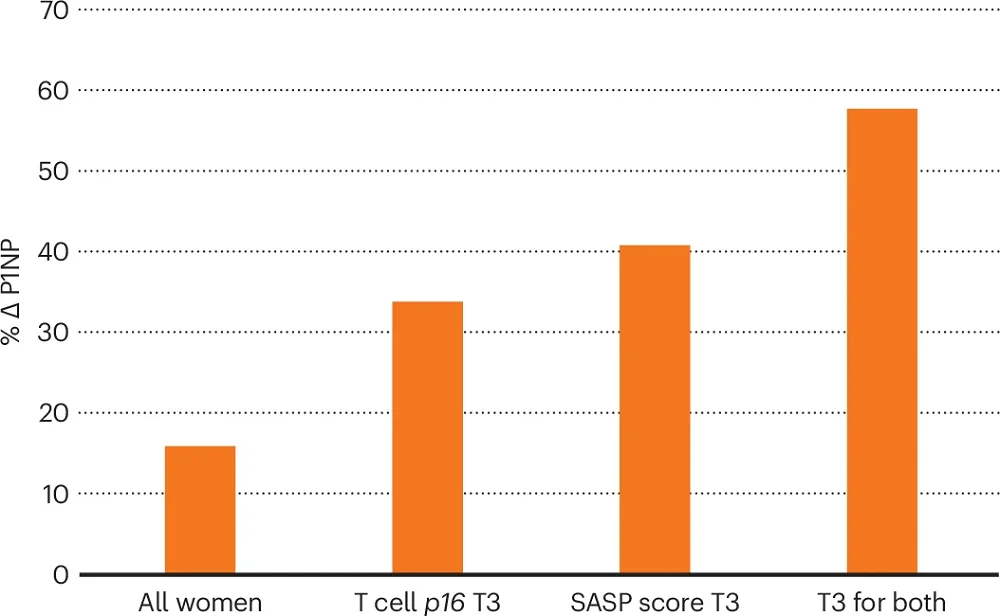
The authors note that while the levels of p16 in T cells appear to be helpful in grouping patients in clinical trials, there is a need to understand the underlying biology behind this connection and assess the variability of p16 levels over time and their correlation with the SASP.
The authors hypothesize that the relationship between senescent cell burden and senolytics might be even more complex. They elaborate that senescent cell burden increases as we age and during diseases. They suggest that the first-generation senolytics, such as dasatinib and quercetin, which have modest potency, are effective in people with high senescent cell burdens, and they hypothesize that second-generation, more potent senolytics (currently under development) should also be effective in people with lower senescent cell burdens. Their hypothesis should be tested once those compounds are developed.
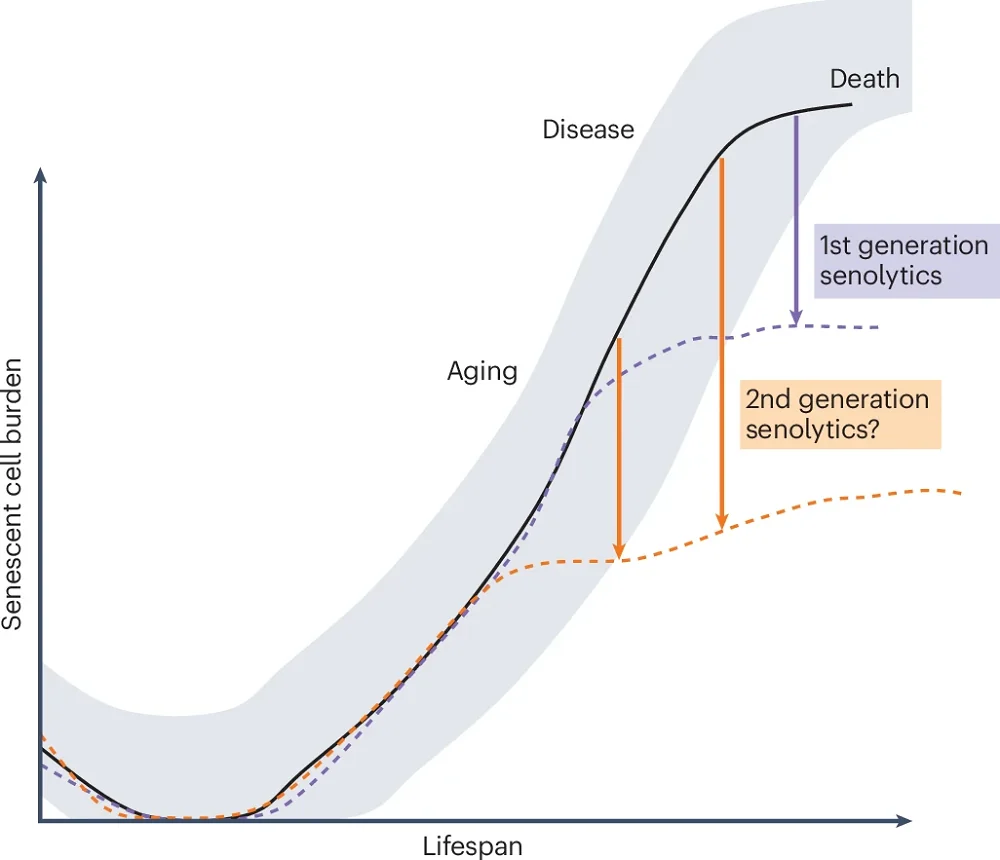
Developing resistance
The authors also point to another unresolved issue that their study identified. They observed the peak of increase in the bone formation marker serum P1NP at week four after starting the dasatinib plus quercetin treatment, but later those levels returned to baseline. One possible explanation can be an induction of compensatory mechanisms. However, the authors also suggest an alternative explanation. They suggest that after initial removal of a subset of senescent cells, remaining cells develop resistance to senolytics. Such a mechanism has never been reported in clinical trials; however, they believe it needs consideration while designing future senolytics clinical trials. The authors suggest two possible approaches that can be applied in future trials to address such an issue: either extending the time between dosing to prevent the development of resistance or incorporating combined treatment with another senolytic that targets different molecular pathways.
The personalized medicine of the future
The authors’ observations warrant further investigation into personalized approaches during senolytics treatment. Since multiple mechanisms drive aging, it is essential to identify the people for whom senescence is a major factor in aging, such as people with high senescent cell burdens, as they might benefit the most from senolytics treatment.
Further, the authors recommend future research that focuses on developing improved biomarkers of senescent cell burden. They believe that the currently used measurement of T cell p16 expression levels or the SASP score they developed, which should still be validated for outcomes unrelated to the skeleton, should be expanded. They propose using large-scale omics-based approaches to develop organ-specific biomarkers for senescent cell burden, which would aid in selecting clinical trial participants with a high senescent cell burden in any particular study’s relevant organ.
In summary, the authors recommend that future clinical trials of senolyics focus on better biomarkers of senescent cell burden and test whether individuals identified with those markers as having high senescent cell burdens indeed respond best to senotherapeutic therapies.
Literature
[1] Khosla, S., Monroe, D. G., & Farr, J. N. (2025). Towards a personalized approach in senolytic trials. Nature aging, 10.1038/s43587-025-00964-5. Advance online publication.
[2] Farr, J. N., Atkinson, E. J., Achenbach, S. J., Volkman, T. L., Tweed, A. J., Vos, S. J., Ruan, M., Sfeir, J., Drake, M. T., Saul, D., Doolittle, M. L., Bancos, I., Yu, K., Tchkonia, T., LeBrasseur, N. K., Kirkland, J. L., Monroe, D. G., & Khosla, S. (2024). Effects of intermittent senolytic therapy on bone metabolism in postmenopausal women: a phase 2 randomized controlled trial. Nature medicine, 30(9), 2605–2612.








