Nanoparticles Potently Reverse Alzheimer’s in Mice
- These polymersomes manage how amyloid beta is processed.

- These nanoparticles guide LRP1, an amyloid-binding protein, to where it needs to be.
- This restores vascular function in Alzheimer’s model mice, allowing them to clear amyloid beta, and significantly ameliorates their cognitive decline.
Scientists have created polymersomes, a type of nanoparticle, that latch onto a master regulator of amyloid-beta clearance, diverting it towards a more efficient route. The treatment drained Aβ from mouse brains within hours and, after a short dosing regimen, restored cognition to near wild-type levels [1].
Get it out of your head
Since the discovery of Alzheimer’s disease, the bulk of scientific attention was devoted to the characteristic plaques that Alois Alzheimer saw in his microscope: large clumps of the misfolded protein amyloid beta (Aβ). Recent research, however, suggests that soluble Aβ monomers, oligomers, and small assemblies can be an even more important factor in the disease’s development [2].
Scientists have devised various ways to clear Aβ from the brain. Two antibody-based therapies have been approved for clinical use, but their efficacy remains limited. A new study from an international team co-led by the Institute for Bioengineering of Catalonia (IBEC) and the West China Hospital Sichuan University (WCHSU), published in Signal Transduction and Targeted Therapy Journal, suggests a new, surprisingly effective approach.
Opening the tunnel
Shuttling soluble Aβ from the brain’s interstitial liquid, through the blood-brain barrier (BBB), and into the bloodstream is regulated by the protein LRP1, which recognizes Aβ, binds to it, and ferries it further. It all, however, depends on avidity: the force with which ligands bind to LRP1 [3]. If avidity is low, little binding occurs.
In Alzheimer’s, however, the problem is the opposite: soluble Aβ aggregates tend to exhibit high avidity towards LRP1 (cling to it tightly). When this happens, the entire construct enters the endothelial cell, a building block of the blood-brain barrier, but never reaches the other side. Instead, both Aβ and the LRP1 molecules get degraded in lysosomes through the Rab5/lysosome pathway. This creates a shortage of available LRP1 molecules and further hampers Aβ removal.
Mid-avidity is the sweet spot: when this is the case, LRP1 recruits another protein, PACSIN2, which curves the endothelial cell’s membrane into a tubular “tunnel” that goes all the way through the cell. Both LRP1 and its toxic cargo then emerge on the other side, and Aβ is deposited into the bloodstream for degradation, while LRP1 returns to its “battle station,” keeping Aβ removal smooth and efficient.
To achieve this optimal state, the researchers created polymersomes studded with ligands for LRP1 and fine-tuned for mid-avidity. By clustering LRP1 just-right, they bias trafficking toward PACSIN2 tubules, restore LRP1 at the vessel wall, and boost Aβ efflux.
Fast-working, long-lasting
The researchers tested their invention in a mouse model of Alzheimer’s disease (APP/PS1). These mice’s brains quickly accumulate Aβ, causing significant cognitive decline. The nanoparticles reduced PET Aβ signal by ~46% at 12 hours and cut 3D-mapped brain Aβ volume by ~41% across 14 regions, while raising plasma/vascular Aβ, consistent with rapid brain-to-blood efflux.
After just three daily IV doses and a seven-day recovery, the treated mice showed drastic improvement on several cognitive tasks. On some of them, such as search efficiency in the Morris water maze, the results were largely indistinguishable from those of wild-type controls.
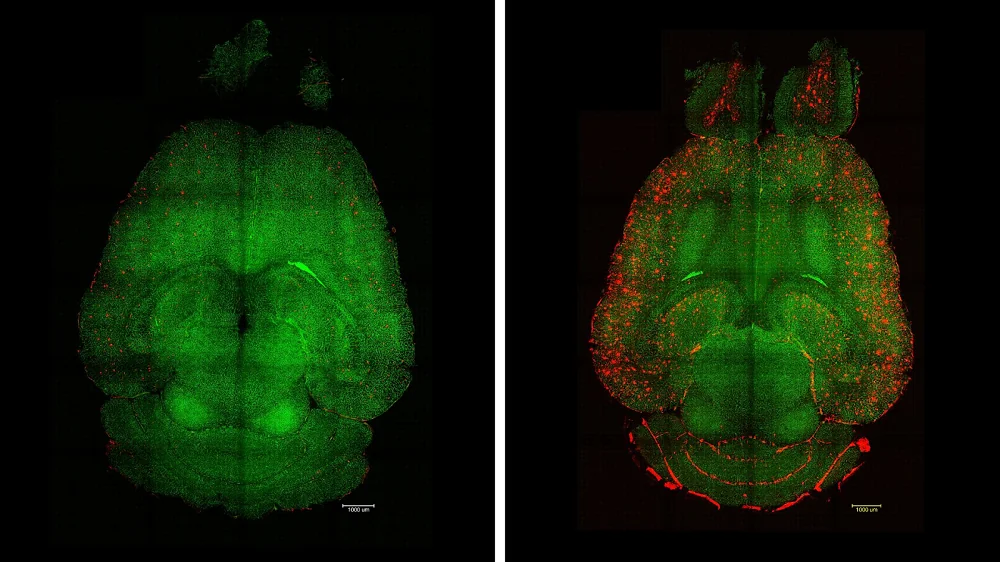
The brain of a treated (left) and untreated (right) mouse 12 hours after an injection. Red dots show Aβ accumulation.
Importantly, the effect proved to be highly durable, indicating a successful reset of BBB function. At six months post-injection, treated APP/PS1 mice again found the platform in the water maze faster, stayed longer at the correct site in the probe, and their overall cognitive performance was similar to that of wild-type mice and significantly better than the control group’s.
The paper frames the platform as a shift from passive shuttles to “supramolecular regulators” that repair BBB transport – a blueprint for neurovascular medicine beyond Alzheimer’s. It is worth noting, however, that mouse models of Alzheimer’s are flawed and validation in humans is needed.
“The long-term effect comes from restoring the brain’s vasculature,” said Giuseppe Battaglia, ICREA Research Professor at IBEC, Principal Investigator of the Molecular Bionics Group and leader of the study. “We think it works like a cascade: when toxic species such as amyloid-beta accumulate, disease progresses. But once the vasculature is able to function again, it starts clearing Aβ and other harmful molecules, allowing the whole system to recover its balance. What’s remarkable is that our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels.”
“Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance, restoring healthy function in the blood–brain barrier and leading to a striking reversal of Alzheimer’s pathology,” added Lorena Ruiz Perez, researcher at the Molecular Bionics group from the Institute for Bioengineering of Catalonia (IBEC) and Serra Hunter Assistant Professor in the Faculty of Physics at the University of Barcelona (UB).
Literature
[1] Chen, J., Xiang, P., Duro-Castano, A., Cai, H., Guo, B., Liu, X., Yu, Y., Lui, S., Luo, K., Ke, B., Ruiz-Pérez, L., Gong, Q., Tian, X., & Battaglia, G. (2025). Rapid amyloid-β clearance and cognitive recovery through multivalent modulation of blood-brain barrier transport. Signal transduction and targeted therapy, 10(1), 331.
[2] Benilova, I., Karran, E., & De Strooper, B. (2012). The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nature neuroscience, 15(3), 349-357.
[3] Tian, X., Leite, D. M., Scarpa, E., Nyberg, S., Fullstone, G., Forth, J., … & Battaglia, G. (2020). On the shuttling across the blood-brain barrier via tubule formation: Mechanism and cargo avidity bias. Science advances, 6(48), eabc4397.







