How Senescent Cells Encourage Melanoma Growth
- A SASP secretion actively encourages melanoma to grow towards it.
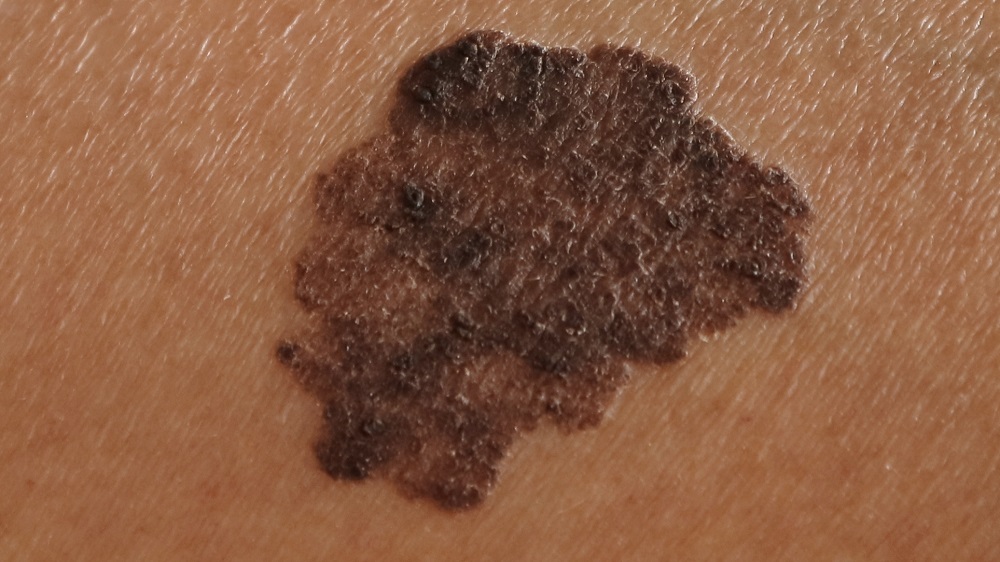
- Melanoma is at its most dangerous when it grows vertically.
- This vertical growth can be driven by senescent cells secreting molecules that encourage it, particularly GCL-2.
- Suppressing GCL-2 or preventing its cancer-encouraging effects may be potential therapy options in the near future.
Researchers publishing in Aging Cell have documented a key reason why older people are much more likely to get melanoma.
Why older people have significantly worse melanoma cases
While melanoma is much more treatable now than in the past, it still remains a serious danger. Melanoma can develop resistance to otherwise effective techniques [1], meaning that they only delay instead of permanently stop the disease.
Fortunately, the origins of melanoma are largely well-understood. The first thing that drives most melanoma cases is a point mutation of the BRAF gene [2]. This does not trigger melanoma by itself, but further mutations of other genes lead to malignancy [3].
The severity of melanoma is measured by its thickness, as it has been known for over half a century that thicker melanomas are much more dangerous [4]. The cancer’s transition from horizontal to vertical growth is associated with increasing mutation frequency [5], and, unsurprisingly, older people are typically diagnosed with considerably thicker melanomas than younger people are [6].

Read More
The researchers of this study have pinpointed cellular senescence as the most likely driver of this increase in severity, and significant previous work has been done to establish the connection between these cells’ potentially dangerous SASP secretions and melanoma [7]. However, that previous work did not completely elucidate the biological relationship between the two, which is what this study was created to find.
Melanoma is attracted to senescent cells
In their first experiment, the researchers confirmed a direct relationship between the prevalence of senescent skin fibroblasts and the incidence of melanoma. While p16 is a tumor suppressor and appears in both the benign and malignant portions of melanoma, it is bypassed by other cancerous mutations [8]. Injecting melanoma cells together with senescent or non-senescent fibroblasts into the skin of mice confirmed this relationship: the mice given senescent fibroblasts had tumors that were ten times as thick.
The researchers then looked into why this is the case. Cultivating melanoma in conditioned media that contains secretions from senescent fibroblasts, but not non-senescent fibroblasts, greatly increases the cancer’s growth. The researchers found two compounds of particular interest: GCL-2 and ENA-78, which melanoma cells were discovered to actively grow towards in a chemotaxic response, resulting in independent, unanchored growth. Neutralizing these compounds in conditioned media through antibodies greatly reduces the growth of melanoma, and enhancing GCL-2 production in non-senescent cells causes their related conditioned media to encourage the growth of this cancer.
These two compounds, which are considerably more abundant in the skin of older adults compared to young adults, are generated by senescent fibroblasts and not significantly by other cell types.
Further work found that GCL-2 is considerably more important than ECL-78 in encouraging the growth of cancer cells, as silencing GCL-2 had significant effects on the growth of melanoma in mice but silencing ECL-78 did not. Furthermore, senescent fibroblasts were confirmed to be the source of the harmful GCL-2, as silencing this compound in the melanoma itself had no significant effect.
The protein that drives melanoma’s growth
A more in-depth examination found this to be due to the phosphorylation of the cAMP-responsive element binding protein (CREB). CREB activation leads to tumor progression in melanoma, activating several related cancer genes, and GCL-2 was found to significantly drive this effect, with ECL-78’s effect being much weaker. Removing GCL-2 from the environment of melanoma was found to almost completely stop CREB activation, both in conditioned media and in mice.
The researchers found that significant CREB activation occurs only in the malignant part of melanoma, not the benign part. It drives glycolysis, a form of energy use that encourages cancer progression. Directly inhibiting this process, either by suppressing CREB or suppressing glycolysis in these cells, prevents the related acceleration of cancer, thus providing strong evidence that this is indeed the biological cause. An examination of naturally occurring melanomas confirmed their glycolytic nature.
As the researchers note, their data “allows several options for novel strategies for therapeutic intervention.” While fighting fibroblast senescence itself would be an ideal solution, targeting GCL-2 or its receptors offers a few potential avenues, and directly targeting CREB offers another. As targeting GCL-2 receptors is already being investigated in the context of other cancers [9], it may be that translating these drugs to melanoma is on the short-term horizon.
Literature
[1] Kim, K. B., Kefford, R., Pavlick, A. C., Infante, J. R., Ribas, A., Sosman, J. A., … & Lewis, K. D. (2013). Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. Journal of Clinical Oncology, 31(4), 482-489.
[2] Gray-Schopfer, V. C., Dias, S. D. R., & Marais, R. (2005). The role of B-RAF in melanoma. Cancer and Metastasis Reviews, 24(1), 165-183.
[3] Dankort, D., Curley, D. P., Cartlidge, R. A., Nelson, B., Karnezis, A. N., Damsky Jr, W. E., … & Bosenberg, M. (2009). Braf V600E cooperates with Pten loss to induce metastatic melanoma. Nature genetics, 41(5), 544-552.
[4] Breslow, A. (1970). Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Annals of surgery, 172(5), 902.
[5] Greene, V. R., Johnson, M. M., Grimm, E. A., & Ellerhorst, J. A. (2009). Frequencies of NRAS and BRAF mutations increase from the radial to the vertical growth phase in cutaneous melanoma. Journal of investigative dermatology, 129(6), 1483-1488.
[6] Kruijff, S., Bastiaannet, E., Francken, A. B., Schaapveld, M., Van Der Aa, M., & Hoekstra, H. J. (2012). Breslow thickness in the Netherlands: a population-based study of 40 880 patients comparing young and elderly patients. British journal of cancer, 107(3), 570-574.
[7] Liu, J., Zheng, R., Zhang, Y., Jia, S., He, Y., & Liu, J. (2023). The cross talk between cellular senescence and melanoma: From molecular pathogenesis to target therapies. Cancers, 15(9), 2640.
[8] Mooi, W. J., & Peeper, D. S. (2006). Oncogene-induced cell senescence—halting on the road to cancer. New England Journal of Medicine, 355(10), 1037-1046.
[9] Campbell, L. M., Maxwell, P. J., & Waugh, D. J. (2013). Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer. Pharmaceuticals, 6(8), 929-959.







