How Inflammation Is Linked to Heart Disease
- Inflammation does damage to both the heart and the vasculature.

- Malfunctioning immune cells in the heart can cause damage.
- Inflammatory cytokines in the vasculature can also lead to deadly clots.
- As inflammation has multiple upstream sources, prevention strategies often involve affecting one or more of these sources.
In Cell Reports Medicine, researchers have published a detailed review on the relationship between cardiovascular disease and the age-related inflammation known as inflammaging.
The immune system itself ages
Read More
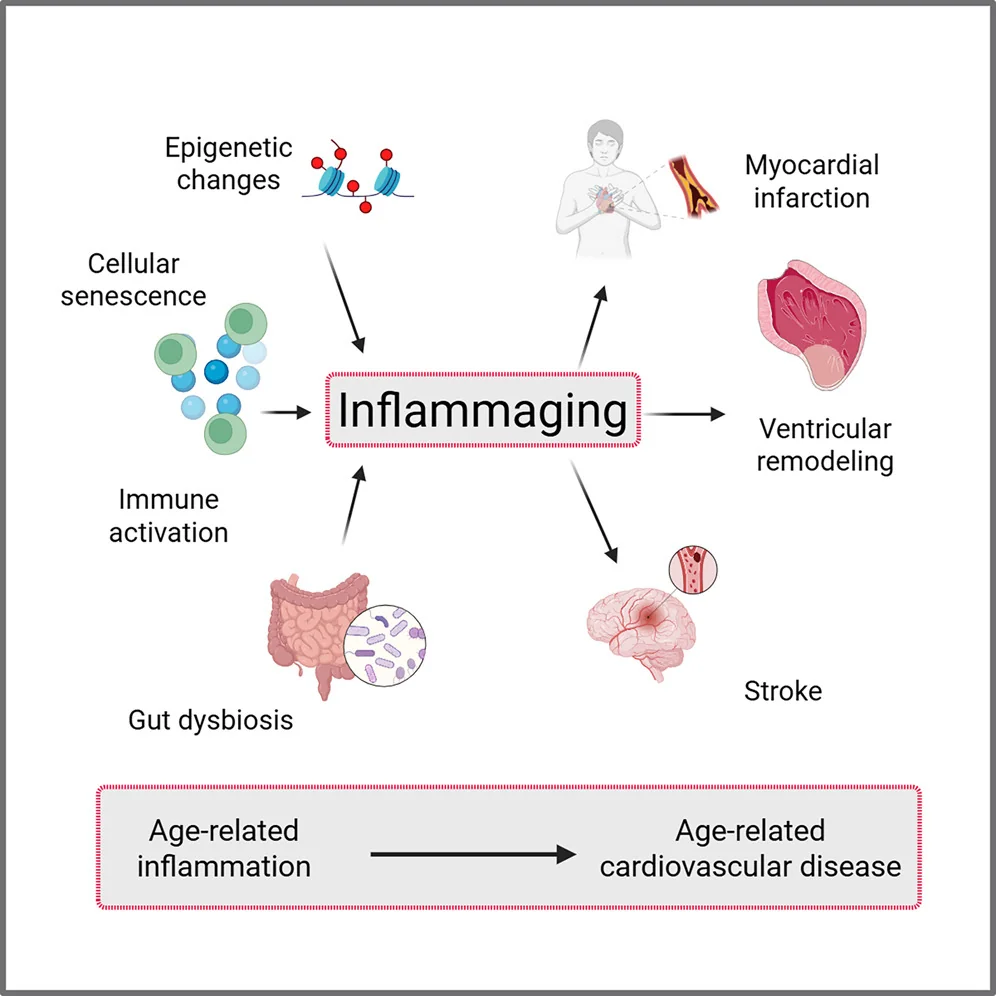
Inflammaging occurs in both the cells sending out signals and in the cells receiving them. The numbers of specific immune cell types become imbalanced; the bone marrow begins producing more myeloid than lymphoid cells [1], leading to an alteration in the neutrophil-to-lymphocyte ratio, which itself is associated with frailty [2].
This imbalance even occurs in the heart. The self-sustaining population of CCR2- macrophages, which promote muscle growth and fight inflammation, is gradually replaced with CCR2+ macrophages, which have a different bodily origin and promote inflammation instead [3].
The thymus is where immune cells are trained, and thymic involution is the age-related process that gradually deteriorates this organ into fat. Mitochondrial dysfunction occurs in the T cells as well, causing further immune dysfunction [4].
Immune dysfunction leads directly to cardiovascular disease
Causing mitochondrial dysfunction in the T cells of mice gave them severe heart disease [5]. The contribution of T cell dysfunction to vascular problems is strong enough that depleting CD8+ T cells in aged mice reduced their atherosclerosis [6]. Similarly, T cells are modified in heart failure, and not for the better; mice that lack CD4+ T cells have better outcomes when subjected to artificial heart attacks [7], and a different study demonstrated that taking T cells from mice subjected to these heart attacks and giving them to other mice causes heart problems in the other mice [8]. Further work confirmed that dysregulated T cells cause long-term damage in this way [9].
T cells even appear to be responsible for the damage caused by well-known inducers of heart disease. As expected, feeding mice a high-fat diet and inducing hypertension causes a form of heart failure in mice, but this does not occur if the mice had their T cells depleted [10].
Inflammation also leads to problems in the vasculature. High levels of circulating inflammatory cytokines lead to endothelial dysfunction, a core contributor to atherosclerosis [11]. This immune overactivation encourages the formation of blood clots (thrombosis) [12], thus increasing the risk of heart attack and stroke [13].
Potential solutions
Unsurprisingly, reducing inflammation is being explored as a method of decreasing the likelihood of thrombotic events [14]. In the 2000s, trials of anti-inflammatory drugs specifically for preventing heart failure did not yield good results [15], but later on, colchichine was found to be successful [16], and a meta-analysis provided enough data for its effectiveness [17] that the FDA has approved it for the prevention of cardiovascular disease in people with multiple risk factors. However, even this drug does not help immediately after a heart attack [18].
Affecting cellular senescence has also been investigated as a potential solution. The link between senescence and inflammation is well-known; the circulating cytokines that can lead to dysfunction are part of the senescence-associated secretory phenotype (SASP), the signals that senescent cells emit [19]. However, fighting senescence to reduce heart problems can carry its own risks; for example, while the combination of dasatinib and quercetin is well-known as a senolytic that destroys senescent cells, dasatinib has been linked to heart problems [20]. Navitoclax, another well-known senolytic, can cause uncontrolled bleeding [21].
The researchers suggest that other drugs that modulate rather than kill senescent cells (senomorphics) may be more promising. Metformin, for example, has been found to reduce the SASP in this way [22]. This may be due to its effects on mitochondrial dysfunction; a study with a different drug suggests that reducing mitochondrial dysfunction by increasing mitochondrial turnover (mitophagy) has beneficial effects in this regard [23].
Some inflammation comes from the gut. The researchers consider well-known interventions such as probiotics, which directly provide healthy gut bacteria [24], along with prebiotics, which feed only these beneficial bacteria [25]. Combining these approaches has demonstrated benefits in pigs with cardiometabolic syndrome [26]. Directly transferring gut bacteria through fecal microbiome transplantation has demonstrated benefits in mice with heart problems [27].
Personalized medicine
The researchers suggest that the detailed relationship between inflammaging and vasculature makes personalized medicine the most preferable approach. Not all preventatives work on everyone with cardiovascular risk factors; for example, one study found that statins don’t offer benefits for people who do not show calcium on CT scans [28]. Similarly, while reducing blood pressure is a common choice to prevent cardiovascular events, antihypertensive drugs can have negative effects on some older people [29]. Advanced imaging and more in-depth examination of biomarkers may allow for more targeted treatments that lead to better outcomes.
Literature
[1] Shaw, A. C., Goldstein, D. R., & Montgomery, R. R. (2013). Age-dependent dysregulation of innate immunity. Nature reviews immunology, 13(12), 875-887.
[2] Dillon, K., Goodman, Z. T., Kaur, S. S., Levin, B., & McIntosh, R. (2023). Neutrophil-to-lymphocyte ratio amplifies the effects of aging on decrements in grip strength and its functional neural underpinnings. The Journals of Gerontology: Series A, 78(6), 882-889.
[3] Bajpai, G., Schneider, C., Wong, N., Bredemeyer, A., Hulsmans, M., Nahrendorf, M., … & Lavine, K. J. (2018). The human heart contains distinct macrophage subsets with divergent origins and functions. Nature medicine, 24(8), 1234-1245.
[4] Thapa, P., & Farber, D. L. (2019). The role of the thymus in the immune response. Thoracic surgery clinics, 29(2), 123-131.
[5] Desdín-Micó, G., Soto-Heredero, G., Aranda, J. F., Oller, J., Carrasco, E., Gabandé-Rodríguez, E., … & Mittelbrunn, M. (2020). T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science, 368(6497), 1371-1376.
[6] Tyrrell, D. J., Wragg, K. M., Chen, J., Wang, H., Song, J., Blin, M. G., … & Goldstein, D. R. (2023). Clonally expanded memory CD8+ T cells accumulate in atherosclerotic plaques and are pro-atherogenic in aged mice. Nature aging, 3(12), 1576-1590.
[7] Yang, Z., Day, Y. J., Toufektsian, M. C., Xu, Y., Ramos, S. I., Marshall, M. A., … & Linden, J. (2006). Myocardial infarct–sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation, 114(19), 2056-2064.
[8] Maisel, A., Cesario, D., Baird, S., Rehman, J., Haghighi, P., & Carter, S. (1998). Experimental autoimmune myocarditis produced by adoptive transfer of splenocytes after myocardial infarction. Circulation research, 82(4), 458-463.
[9] Bansal, S. S., Ismahil, M. A., Goel, M., Zhou, G., Rokosh, G., Hamid, T., & Prabhu, S. D. (2019). Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation, 139(2), 206-221.
[10] Smolgovsky, S., Bayer, A. L., Kaur, K., Sanders, E., Aronovitz, M., Filipp, M. E., … & Alcaide, P. (2023). Impaired T cell IRE1α/XBP1 signaling directs inflammation in experimental heart failure with preserved ejection fraction. The Journal of clinical investigation, 133(24).
[11] Sprague, A. H., & Khalil, R. A. (2009). Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical pharmacology, 78(6), 539-552.
[12] Riegger, J., Byrne, R. A., Joner, M., Chandraratne, S., Gershlick, A. H., Ten Berg, J. M., … & Zahman, A. (2016). Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. European heart journal, 37(19), 1538-1549.
[13] Liu, Y., Guan, S., Xu, H., Zhang, N., Huang, M., & Liu, Z. (2023). Inflammation biomarkers are associated with the incidence of cardiovascular disease: a meta-analysis. Frontiers in Cardiovascular Medicine, 10, 1175174.
[14] Eikelboom, J. W., Connolly, S. J., Bosch, J., Dagenais, G. R., Hart, R. G., Shestakovska, O., … & Yusuf, S. (2017). Rivaroxaban with or without aspirin in stable cardiovascular disease. New England Journal of Medicine, 377(14), 1319-1330.
[15] Chung, E. S., Packer, M., Lo, K. H., Fasanmade, A. A., & Willerson, J. T. (2003). Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation, 107(25), 3133-3140.
[16] Tardif, J. C., Kouz, S., Waters, D. D., Bertrand, O. F., Diaz, R., Maggioni, A. P., … & Roubille, F. (2019). Efficacy and safety of low-dose colchicine after myocardial infarction. New England journal of medicine, 381(26), 2497-2505.
[17] Fiolet, A. T., Poorthuis, M. H., Opstal, T. S., Amarenco, P., Boczar, K. E., Buysschaert, I., … & Kelly, P. J. (2024). Colchicine for secondary prevention of ischaemic stroke and atherosclerotic events: a meta-analysis of randomised trials. EClinicalMedicine, 76.
[18] Jolly, S. S., d’Entremont, M. A., Lee, S. F., Mian, R., Tyrwhitt, J., Kedev, S., … & Yusuf, S. (2025). Colchicine in acute myocardial infarction. New England Journal of Medicine, 392(7), 633-642.
[19] Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., Morton, J. P., … & Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology, 15(8), 978-990.
[20] Xu, Z., Cang, S., Yang, T., & Liu, D. (2009). Cardiotoxicity of tyrosine kinase inhibitors in chronic myelogenous leukemia therapy. Hematology Reviews, 1(1), e4.
[21] Schoenwaelder, S. M., Jarman, K. E., Gardiner, E. E., Hua, M., Qiao, J., White, M. J., … & Jackson, S. P. (2011). Bcl-xL–inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood, The Journal of the American Society of Hematology, 118(6), 1663-1674.
[22] Abdelgawad, I. Y., Agostinucci, K., Sadaf, B., Grant, M. K., & Zordoky, B. N. (2023). Metformin mitigates SASP secretion and LPS-triggered hyper-inflammation in Doxorubicin-induced senescent endothelial cells. Frontiers in Aging, 4, 1170434.
[23] Kelly, G., Kataura, T., Panek, J., Ma, G., Salmonowicz, H., Davis, A., … & Korolchuk, V. I. (2024). Suppressed basal mitophagy drives cellular aging phenotypes that can be reversed by a p62-targeting small molecule. Developmental cell, 59(15), 1924-1939.
[24] Wierzbicka, A., Mańkowska-Wierzbicka, D., Mardas, M., & Stelmach-Mardas, M. (2021). Role of probiotics in modulating human gut microbiota populations and activities in patients with colorectal cancer—a systematic review of clinical trials. Nutrients, 13(4), 1160.
[25] Yoo, S., Jung, S. C., Kwak, K., & Kim, J. S. (2024). The role of prebiotics in modulating gut microbiota: implications for human health. International Journal of Molecular Sciences, 25(9), 4834.
[26] Herisson, F. M., Cluzel, G. L., Llopis-Grimalt, M. A., O’Donovan, A. N., Koc, F., Karnik, K., … & Caplice, N. M. (2025). Targeting the gut-heart axis improves cardiac remodeling in a clinical scale model of cardiometabolic syndrome. Basic to Translational Science, 10(1), 1-15.
[27] Hatahet, J., Cook, T. M., Bonomo, R. R., Elshareif, N., Gavini, C. K., White, C. R., … & Aubert, G. (2023). Fecal microbiome transplantation and tributyrin improves early cardiac dysfunction and modifies the BCAA metabolic pathway in a diet induced pre-HFpEF mouse model. Frontiers in cardiovascular medicine, 10, 1105581.
[28] Mitchell, J. D., Fergestrom, N., Gage, B. F., Paisley, R., Moon, P., Novak, E., … & Villines, T. C. (2018). Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. Journal of the American College of Cardiology, 72(25), 3233-3242.
[29] Benetos, A., Petrovic, M., & Strandberg, T. (2019). Hypertension management in older and frail older patients. Circulation research, 124(7), 1045-1060.







