A Potential Reason Why Clotting Increases With Age
- Aged cells are likely to create more reactive platelets.
- Older mice create more platelet-forming cells via a non-canonical pathway.
- Platelets created by cells that originated from this pathway are more highly reactive than normal platelets, and the cells are more likely to survive.
- Researchers have identified a way to determine whether cells of this type were created through the canonical or non-canonical process.
In Aging Cell, researchers have described a method by which platelet-forming cells are rapidly generated from hematopoietic stem cells (HSCs), bypassing the intermediate cell types that are normally used to get there.
Canonical and non-canonical formation
It is well known that blood clots, which form when platelets bind together, are a serious problem in older people [1]. Arterial clots directly cause both heart attacks and strokes, often being the final capstones on gradual cardiovascular aging, but changes in HSC behavior drive many other conditions as well [2].
In the canonical pathway, HSCs go through three intermediate cell types before finally becoming megakaryocyte progenitors (MkPs), which form the megakaryocytes that release platelets [3]. This team has previously discovered that a non-canonical pathway also exists and increases with age, turning HSCs directly into MkPs with no intermediate steps [4]. Worse, this previous work found that platelets created by non-canonical MkPs (ncMkPs) are hyperactive, heightening their potential contributions to clotting-related diseases.
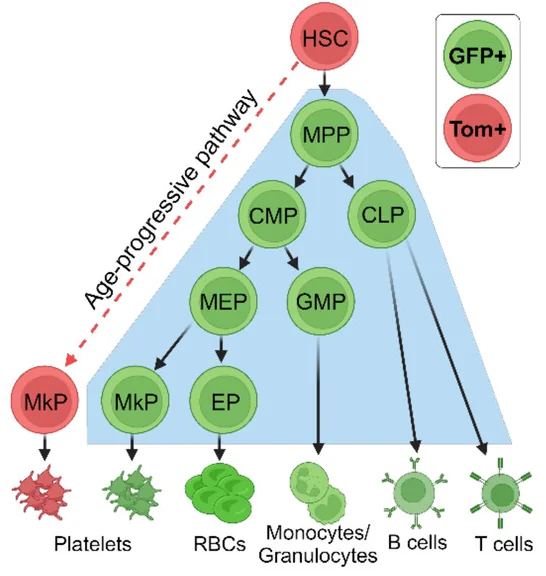
To illustrate these differences, the researchers had created a mouse model, FlkSwitch, that expresses green fluorescent protein (GFP) in MkPs that go through the canonical pathway and a different fluorescent protein, Tomato, in MkPs that go through the faster, non-canonical pathway. This paper is a closer examination into the differences between these MkPs, as the researchers wanted to be able to identify them without the need for specialized mice and to determine the functional differences between them.
Finding the right biomarkers
In their first experiment, the researchers combined their previous bulk-sequencing RNA results with single-cell sequencing. They were looking for membrane-bound proteins, which could potentially be used in future studies or even clinical practice to determine which MkPs are which, and they sought proteins for which commercially available antibodies already exist.
Initially, the researchers hit upon CD54 as their target protein, but further work found that CD48 was a better fit; MkPs expressing more CD48 are much more likely to be created through the canonical pathway, as determined by GFP and Tomato comparisons. Another protein, CD321, was found to be a strong predictor in the other direction; MkPs that express high amounts of this protein are much more likely to be created through the non-canonical pathway. This combination of CD48 and CD321 was highly useful in creating a firm basis for differentiating MkPs, as it yielded similar results to those gleaned from the FlkSwitch mice.
A further experiment found that ncMkPs are not entirely exclusive to aged animals. In 2- to 3-month-old male mice, approximately one in five MkPs is created through the non-canonical pathway. In aged male mice, however, this changes to an average of three in five, although there are large variations between animals. For female mice, there was a statistically significant increase with aging, but it was somewhat less noticeable and older female mice have fewer platelets overall than older male mice do, despite having similar numbers of MkPs.
Hardier survivors
One crucial and surprising finding was that older ncMkPs have a much greater ability to survive in vitro than all other MkPs. When derived from younger animals, ncMkPs proliferate poorly, less than canonical MkPs (cMkPs) of any age. Older ncMkPs, on the other hand, proliferate much more.and are much less likely to die by apoptosis.
This increase in activity persisted in vivo as well; older ncMkPs that were given to younger mice, which had their relevant cells previously destroyed by radiation, proliferated much more than younger ncMkPs did. Additionally, chemical stimulation determined that the platelets produced by older ncMkPs were, indeed, extremely reactive.
The environment plays a role
Taking HSCs from younger and older mice and observing their conversion into MkPs yielded entirely expected results; HSCs derived from the older animals were much more likely to produce ncMkPs. However, these findings changed when these cells were introduced into a younger irradiated animal; there, HSCs produced similar proportions of MkPs, regardless of whether they had come from a younger or older source. A closer examination to determine if any individual HSC is more or less likely to prouce ncMkPs yielded no useful results, suggesting that the aged environment is a strong factor in HSCs creating ncMkPs
As the researchers point out, however, humans and mice differ, and platelet generation is one of those differences; in humans, platelet count decreases with age [5]. Therefore, it is not clear if these murine findings could directly translate to human beings. However, with the identification of the proteins CD48 and CD321 as biomarkers, further work on human MkPs may reveal whether this change in cell fate is a significant factor in human clot-related diseases along with potential interventions to address it.
Literature
[1] Le Blanc, J., & Lordkipanidzé, M. (2019). Platelet function in aging. Frontiers in cardiovascular medicine, 6, 109.
[2] Chung, S. S., & Park, C. Y. (2017). Aging, hematopoiesis, and the myelodysplastic syndromes. Blood advances, 1(26), 2572-2578.
[3] Manso, B. A., Rodriguez y Baena, A., & Forsberg, E. C. (2024). From hematopoietic stem cells to platelets: Unifying differentiation pathways identified by lineage tracing mouse models. Cells, 13(8), 704.
[4] Poscablo, D. M., Worthington, A. K., Smith-Berdan, S., Rommel, M. G., Manso, B. A., Adili, R., … & Forsberg, E. C. (2024). An age-progressive platelet differentiation path from hematopoietic stem cells causes exacerbated thrombosis. Cell, 187(12), 3090-3107.
[5] Jones, C. I. (2016). Platelet function and ageing. Mammalian genome, 27(7), 358-366.







