DNA origami, a nano-scale technique used to make very tiny smiley faces out of DNA, is becoming increasingly valuable for drug delivery and other applications, paving the way for true nanomedicine.
What is DNA origami?
Ever since Richard Feynman famously proclaimed in his seminal 1959 talk that “there’s plenty of room at the bottom”, nanotechnology has been hailed as the next technological wonder. In reality, though, its progress has remained slow for decades due to the enormous challenges of building and operating structures on a nanometric scale. Thankfully, in recent years, the pace has quickened, showering us with promising developments. This includes one of the most exciting areas of medical nanotechnology: DNA origami.
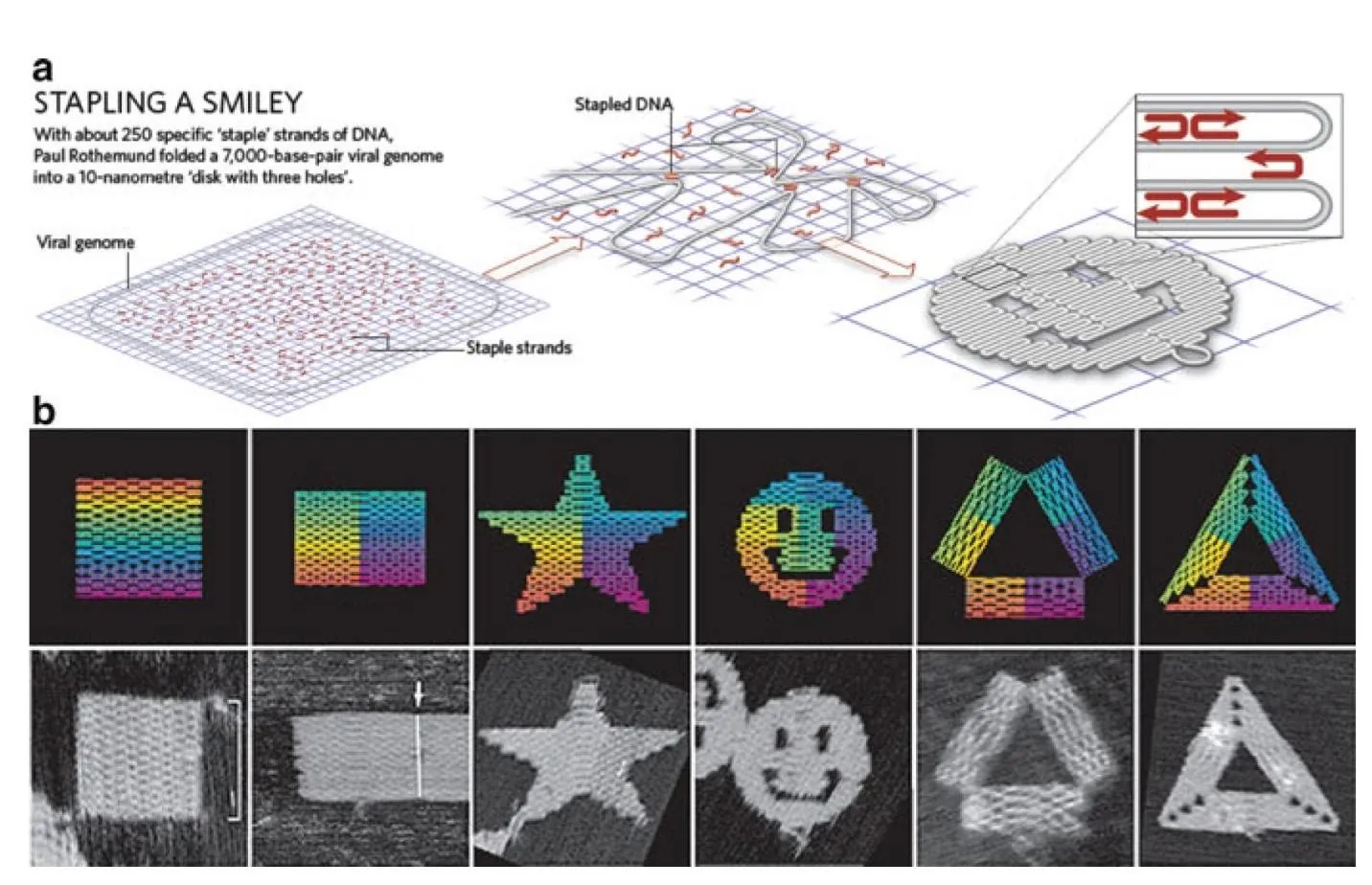
Back in 1982, Nadrian Seeman of New York University, inspired by the mind- and space-bending works of M. C. Escher, pioneered the idea of using DNA molecules to build complex three-dimensional structures. Capitalizing on Seeman’s work more than a decade later, Paul Rothemund of CalTech invented a new way of constructing objects from DNA, which he called “DNA origami”. Like an elaborate origami figurine folded from a single sheet of paper, DNA origami objects are built from one long “scaffold” of single-stranded DNA bound in particular places by “staples” that consist of hundreds of shorter strands. As a scaffold, most DNA origami objects use a single strand of DNA, over 7000 base pairs long, that originates from an M13 bacteriophage virus. The staples are man-made.
The staples have a specific base sequence that can only bond with corresponding sequences on the scaffold (thanks to the Watson-Crick base pairing). When two sections of one staple bond with two different regions of the scaffold DNA, they bring those regions together, not unlike real staples, creating a compact and relatively rigid structure. This structure becomes a tiny part of the desired shape.
One of the main advantages and mysteries of DNA origami is the spontaneous process of folding into shape, which is still not fully understood. The building blocks (billions of scaffolds and staples mixed in precise proportion) are subjected to annealing: heating followed by slow cooling. During this process, preprogrammed shapes are formed automatically, although the yield can be low for complex structures. This ability to self-assemble makes DNA origami probably the most powerful and scalable DNA construction technology to date.
To demonstrate the robustness of his method, Rothemund had created numerous objects, including the now-famous smiley faces that are 10 nanometers in diameter. In the following years, the DNA origami technique has been expanded by Rothemund and others to include an impressive array of much more complex two- and three-dimensional objects [1].
How can this help us live longer?
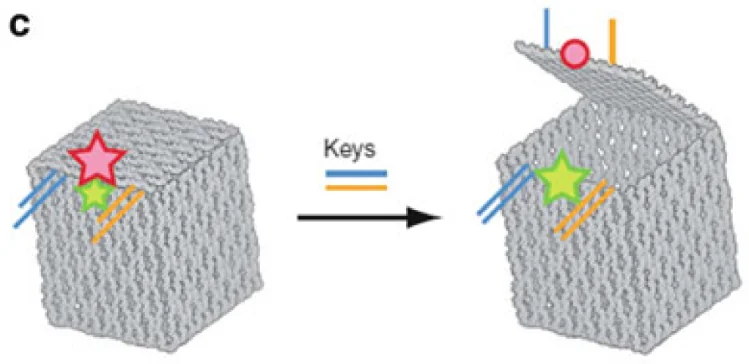
In recent years, researchers have been devising ingenious ways of making the tiny structures exhibit robotic behavior. Some of the resulting designs have demonstrated great potential as delivery vehicles for drug molecules [2]. One example is three-dimensional DNA origami “boxes” made of interconnected two-dimensional plates. Each of these objects has an internal cavity and a door with a molecular lock. Its door can be unlocked by a specific chemical “key” – such as a compound associated with a certain type of cancer cell. DNA origami nanorobots behave like simple logical gates. A robot with two locks, each one reacting to a different compound, functions as an AND gate, opening the internal cavity only when both compounds are present. This allows for more targeted and specialized uses.
The possible applications for targeted action on a cellular scale are innumerable. In 2016, it was demonstrated that DNA nanorobots can be used for diagnostics. A team of scientists used gold nanorods attached to DNA origami structures. After having been absorbed by the tumor cells, the nanorods were used first as contrast agents in optoacoustic imaging (OAI) and then as thermal agents in photothermal therapy. The latter is based on the irradiation of gold particles by a near-infrared laser which leads to their rapid heating. The heat reduces viability of the nearby cancer cells [3].
In 2018, we reported on the successful delivery of thrombin to tumors in mice by DNA origami nanorobots. Thrombin was delivered straight to the tumor in the hope of cutting off its blood supply. The nanorobots succeeded in inhibiting the tumor’s growth and, importantly, were labeled safe after being extensively tested in mice and Bama pigs [4].
Last year, a team from Augmanity Nano and the Wyss Institute for Biologically Inspired Engineering at Harvard reported on nanoscale robots that exhibit quorum sensing (QS). In biology, quorum sensing is the ability of some animals, including bacteria and insects, to adjust their behavior in response to population density fluctuations. The team engineered a QS system to address the problem of drug concentration at the target, as a simpler DNA robot releases its payload immediately. If too few of these simpler robots get to the target simultaneously, drug concentration may not reach the levels necessary for effective treatment. The QS system, however, causes the nanorobots to sense how many of their “siblings” are also in the vicinity of the target and to coordinate a simultaneous payload release [5].
Finally, just last month, a group of Chinese scientists claimed that they were able to create what amounts to a nanoscale precision-guided missile made of DNA (D-PGM) [6]. The robots consist of a warhead loaded with the well-known chemotherapy drug doxorubicin and a guidance system capable of recognizing specific cell subtypes.
The results demonstrate that by mimicking the functionalities of a military precision-guided missile to design the sequential disassembly of the GC system in multistimuli-responsive fashion, our intrinsically biocompatible and degradable D-PGM can accurately identify target cancer cells in complex biological milieu and achieve active targeted drug delivery. The success of this strategy paves the way for specific cell identity and targeted cancer therapy.
This experiment highlights the fact that we already possess capable anti-cancer drugs that may only need targeted delivery systems to unleash their full potential.
Conclusion
While many problems remain, such as the survivability of fragile DNA machines in the body [7], successes continue to mount. Numerous start-ups, such as Tilibit Nanosystems, Gattaquant, Integrated DNA Technologies, and Eurofins Genomics, explore commercial applications and offer custom-built nanostructures for purposes ranging from medicine to quantum computing. A few software tools for designing DNA origami exist, most notably the open-source CadNano. The annual BIOMOD competition features novel DNA origami designs from young teams all over the world. While no clinical trials have been conducted yet, the long-foretold days when we have swarms of nanorobots circulating in our bloodstreams in order to prevent, diagnose, and fight diseases are likely to be on the horizon.
Literature
- Liu, H., & Fan, C. (2013). DNA Origami Nanostructures. In DNA Nanotechnology (pp. 207-224). Springer, Berlin, Heidelberg.
- Udomprasert, A., & Kangsamaksin, T. (2017). DNA origami applications in cancer therapy. Cancer science, 108(8), 1535-1543.
- Du, Y., Jiang, Q., Beziere, N., Song, L., Zhang, Q., Peng, D., … & Ntziachristos, V. (2016). DNA‐Nanostructure–Gold‐Nanorod Hybrids for Enhanced In Vivo Optoacoustic Imaging and Photothermal Therapy. Advanced Materials, 28(45), 10000-10007.
- Li, S., Jiang, Q., Liu, S., Zhang, Y., Tian, Y., Song, C., … & Chang, Y. (2018). A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nature biotechnology, 36(3), 258.
- Amir, Y., Abu-Horowitz, A., Werfel, J., & Bachelet, I. (2019). Nanoscale robots exhibiting quorum sensing. Artificial Life, 25(3), 227-231.
- Ouyang, C., Zhang, S., Xue, C., Yu, X., Xu, H., Wang, Z., … & Wu, Z. S. (2020). Precision guided missile-like DNA nanostructure containing warhead and guidance control for aptamer-based targeted drug delivery into cancer cells in vitro and in vivo. Journal of the American Chemical Society.
- Balakrishnan, D., Wilkens, G. D., & Heddle, J. G. (2019). Delivering DNA origami to cells. Nanomedicine, 14(7), 911-925.




