A new paper published in Nutrients shows that the well-known NAD+ precursor NMN alleviates lung injury caused by silicate inhalation in wild-type mice.
An antioxidant approach to a common problem
Silicosis is an occupational hazard encountered by people who are regularly exposed to silica dust [1]. It is a common cause of lung injury around the world [2]. Silica dust enters cells easily, but cells find it hard to remove [3]; when they do remove it, it is often through phagocytosis, and the released particles are then often consumed once again [4].
One of the main ways that silicosis harms cells is through cellular stress in the forms of inflammation and oxidation, which are problems that NMN has been reported to alleviate in previous studies related to other conditions [5].
Effective against injury and inflammation
For this study, the researchers used five groups of wild-type mice: a sham group, a silicate inhalation group, groups that received high (one gram per kilogram) and low (half a gram per kilogram) doses of NMN along with silica inhalation, and an NMN control group that only received a gram per kilogram of NMN. These mice were only six to eight weeks old.
After one week of this treatment, the results were already statistically significant. The two NMN treatment groups were found to have significantly less scar-related collagen tissue and significantly fewer lesions than the silica-only group.
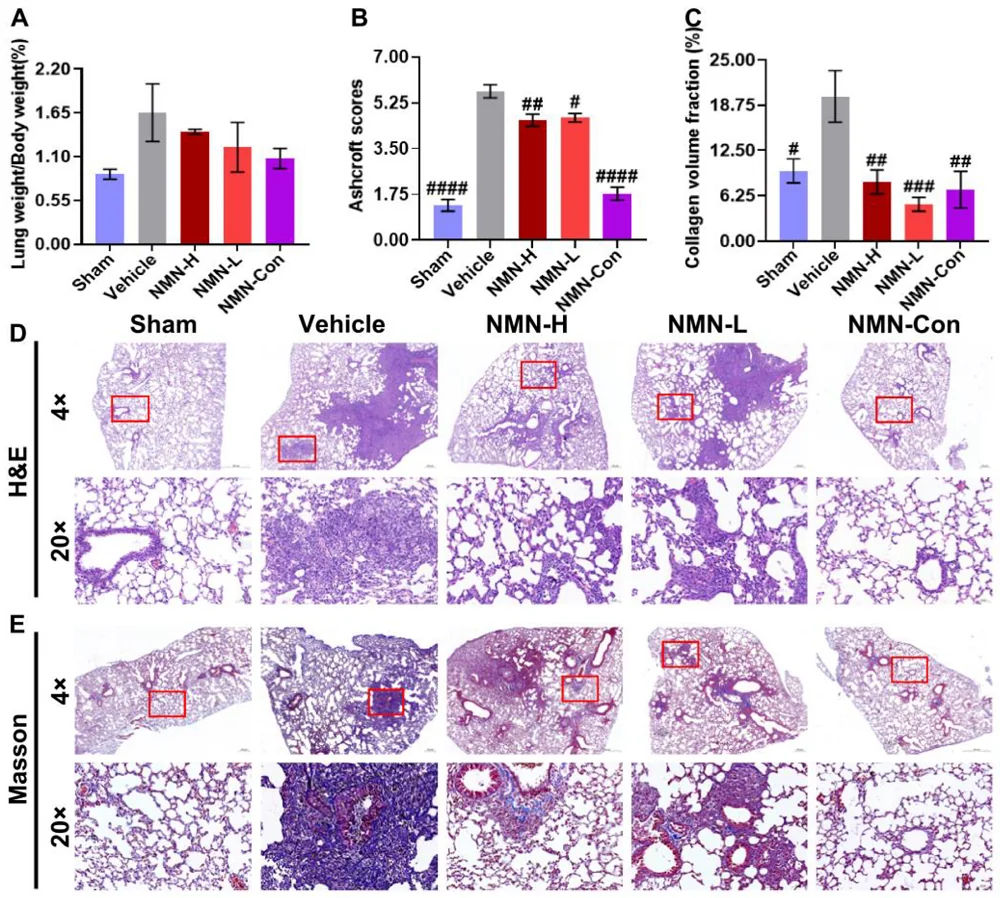
Four weeks showed even more significant results. Lung weight, which had significantly increased among the silica-only group, had not increased nearly as much in the NMN groups, which had significantly reduced collagen as well. While both NMN treatment groups benefited, the high-dose group fared better than the low-dose group in lung lesions.
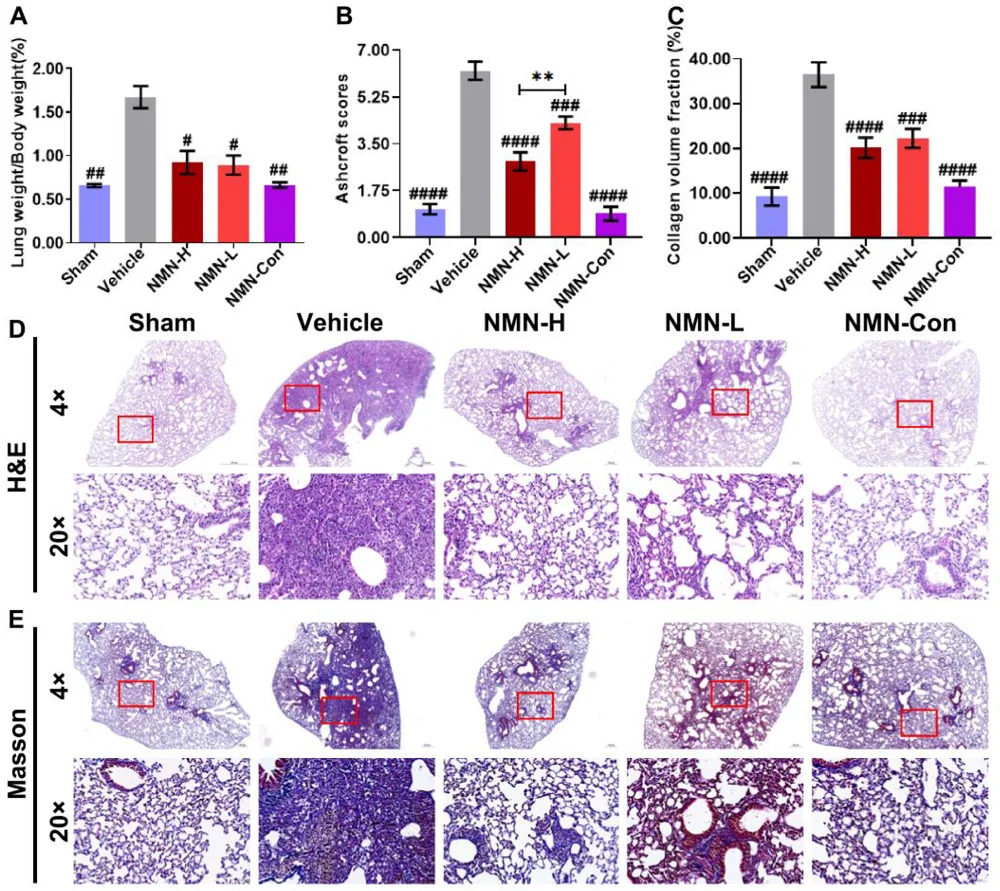
Similar results were reported in reactive oxygen species (ROS), glutathione (GSH), and macrophage count. While low doses of NMN were not shown to be effective against ROS at 7 days, they were effective at 28 days. GSH decreases with silica inhalation but is restored with NMN at low and high doses. Macrophage count increases with silica as well, and this measurement of inflammation is also reduced with NMN, particularly after four weeks.
Gene expression analysis confirmed these findings, showing that GSH-related metabolism, which is mediated by the production of the ROS metabolism factor Nrf2, is a likely cause of these results. In these mice, Nrf2 was found to be decreased with silica inhalation and restored with high-dose NMN. Other genes related to the metabolism of foreign matter and detoxification were found to be decreased with silica and enhanced with NMN at both low and high doses after 28 days.
Conclusion
The researchers note that although they have explored some of the fundamental biology, the lung microenvironment remains largely unexplored. Therefore, despite what has been learned from these experiments, there is not yet a full understanding of how NMN interacts with lung fibroblasts in living tissue.
While this study focuses on environmental exposure rather than intrinsic aging, it is not a leap of logic to suggest that these effects against this particular form of accumulated damage may apply to other forms of accumulated damage as well. However, this is still a mouse study. Further trials are necessary to confirm these results and determine if NMN is effective in alleviating silicosis, or other sources of long-term tissue damage, in human beings.
Literature
[1] Barnes, H., Goh, N. S., Leong, T. L., & Hoy, R. (2019). Silica‐associated lung disease: an old‐world exposure in modern industries. Respirology, 24(12), 1165-1175.
[2] Shi, P., Xing, X., Xi, S., Jing, H., Yuan, J., Fu, Z., & Zhao, H. (2020). Trends in global, regional and national incidence of pneumoconiosis caused by different aetiologies: an analysis from the Global Burden of Disease Study 2017. Occupational and Environmental Medicine, 77(6), 407-414.
[3] Rimola, A., Costa, D., Sodupe, M., Lambert, J. F., & Ugliengo, P. (2013). Silica surface features and their role in the adsorption of biomolecules: computational modeling and experiments. Chemical reviews, 113(6), 4216-4313.
[4] Benmerzoug, S., Rose, S., Bounab, B., Gosset, D., Duneau, L., Chenuet, P., … & Quesniaux, V. F. (2018). STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nature communications, 9(1), 1-19.
[5] Wan, Y., He, B., Zhu, D., Wang, L., Huang, R., Zhu, J., … & Gao, F. (2021). Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Archives of Biochemistry and Biophysics, 712, 109050.