Underdog Pharmaceuticals (Now called Cyclarity Therapeautics) has just issued a press release announcing that UDP-003, a compound being studied for its effectiveness against oxidized cholesterol, has been given an Innovation Passport under the UK’s Innovative Licensing and Access Pathway program. This is similar to the Fast Track designation given by the FDA in the United States.
Underdog is a spin-out company from SENS Research Foundation, and Matthew O’Connor, who is quoted in this article, is well-known for his previous activities at SENS Research Foundation.
Mountain View, CA, U.S., September 8, 2021 –
Underdog Pharmaceuticals, Inc. (Underdog), a pre-clinical stage pharmaceutical company focusing on the treatment and reversal of age-related diseases, has been awarded an Innovation Passport under the United Kingdom’s Innovative Licensing and Access Pathway (ILAP), to pursue fast patient access to its groundbreaking treatment for cardiovascular disease.
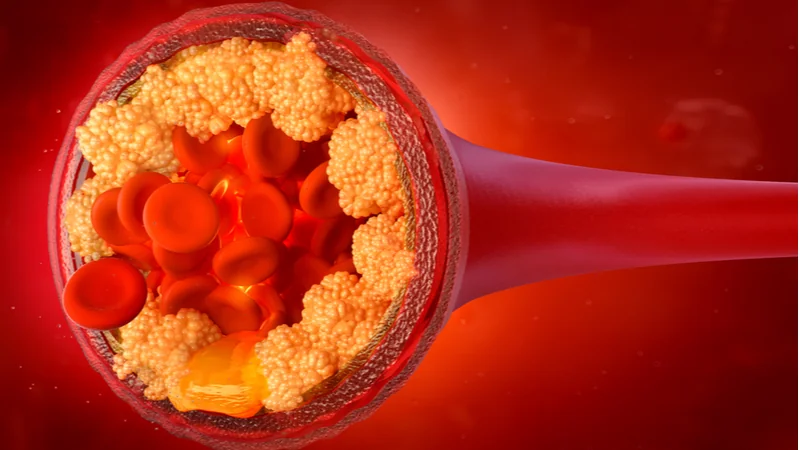
Accounting for more than 40% of deaths in Europe and the United States, and with annual treatment costs more than £29 billion in the UK alone, cardiovascular disease is by far the world’s most life-threatening condition, and its primary cause is atherosclerosis. Underdog’s engineered synthetic carbohydrate compound, UDP-003, is designed to target and remove toxic oxidized cholesterol, a key driver of arterial plaque accumulation. Underdog is one of the first companies, and UDP-003 one of the very few pre-clinical therapies, to receive an ILAP designation.
ILAP, introduced by the Medicines and Healthcare products Regulatory Agency (MHRA) in January, is designed to accelerate development of and access to promising medicines of significant potential public health benefit. The program provides enhanced early input and interactions with MHRA, the National Institute for Health and Care Excellence (NICE), the Scottish Medicines Consortium (SMC), and the National Health Service (NHS). The ILAP program also provides the potential for significant downstream regulatory advantages, including rolling clinical reviews, accelerated assessment, and supervised early reimbursed use under adaptive authorization.
“This is an honor for us and a wonderful step forward for our program,” said Matthew S. O’Connor, Underdog CEO of Scientific Affairs. “There is an enormous public need for drugs which can significantly reverse established atherosclerosis, rather than manage or delay its onset.”
“I believe the MHRA is sending a powerful message by reviewing and admitting preclinical stage programs like UDP-003 into ILAP,” noted Mike Kope, Underdog CEO of Corporate Affairs. “We’re pursuing a new target, with a new compound and seeking evidence of genuine disease modification; and though the potential is fascinating, the clinical challenges are real. The advice and consultation we’ll receive will be invaluable to the success of the program.”
About Underdog
Underdog Pharmaceuticals, Inc., is pursuing a mission to treat the underlying causes of age-related disease. The company develops simple and direct interventions targeting oxidized cholesterol using rationally designed molecules, to provide the first true disease-modifying treatments for common age-related conditions such as atherosclerosis, heart attack and stroke. Its products are based on novel derivatives of a well-known, safe compound and a new way of looking at cardiovascular disease. For more information, please visit www.underdogpharma.com or send an email to [email protected]
Forward Looking Statements
The information and statements contained herein contain forward-looking statements, which are subject to risks and uncertainties that could cause actual results to differ materially from such forward-looking statements and should not be considered as an indication or guarantee of future performance. Forward-looking statements involve known and unknown risks and uncertainties which could cause the Company’s prospects or actual results to differ materially from those in the forward-looking statements. The Company does not undertake any obligation to update the information or forward-looking statements contained herein whether as a result of new information or future events or otherwise.
Contact:
Mike Kope
Co-CEO
Underdog Pharmaceuticals Inc