Scientists have successfully tested a new nanoparticle-based delivery system for CRISPR kits, achieving drastic improvements in mouse models of glioblastoma and ovarian cancer [1].
A gift from our single-cell ancestors
CRISPR gene editing technology has been widely hailed as a potential game changer in medicine, including anti-cancer therapy. CRISPR’s ability to permanently disrupt tumor survival genes potentially constitutes a giant improvement over the long, exhausting, and toxic treatment protocols that exist today. However, to achieve this feat, CRISPR-based molecular systems need to be delivered to every cancer cell, which is not a trivial task.
Scientists gleaned CRISPR from bacteria. In nature, this evolutionarily developed defense mechanism allows bacteria to remember the DNA of bacteriophage viruses it encounters. The data is stored in the cell’s genome in sequences called CRISPR arrays. In the event of a new penetration by the same virus, the CRISPR system recognizes and cuts the viral DNA using Cas (CRISPR-associated proteins), rendering the virus harmless. It is essentially a primitive form of acquired immunity for single-cell organisms.
Several Cas proteins have been found in various bacteria. Cas9 is one of the most promising, since it can cleave nearly any DNA sequence. Unfortunately, it is also massive, making targeted cellular delivery of Cas9-based systems challenging. In previous research, the best results were achieved in the liver, with up to 60% gene editing efficacy (the percentage of cancer cells edited). In cancers that affect other tissues, the results have been less encouraging, such as 15% in lung cancer and 3% in melanoma.
Self-assembling weapons
Most in vivo studies have used adeno-associated viruses (AAVs) to deliver CRISPR components to cells. Unfortunately, this delivery system is plagued by low carrying capacity, hepatotoxicity at high doses, long-term genotoxicity [2], and other effects. It also tends to provoke an immune response, as it is based on viral DNA [3]. This time, researchers decided to try a different approach: lipid nanoparticles (LNPs). In recent years, LNPs have gained popularity as a delivery vehicle for drug molecules [4], due to their superior cell-penetrating abilities and low toxicity. “Loaded” LNPs are assembled from a mixture of lipid molecules and cargo molecules (Cas9-coding mRNA and the “single-guide RNA” that tells Cas9 where to cut):
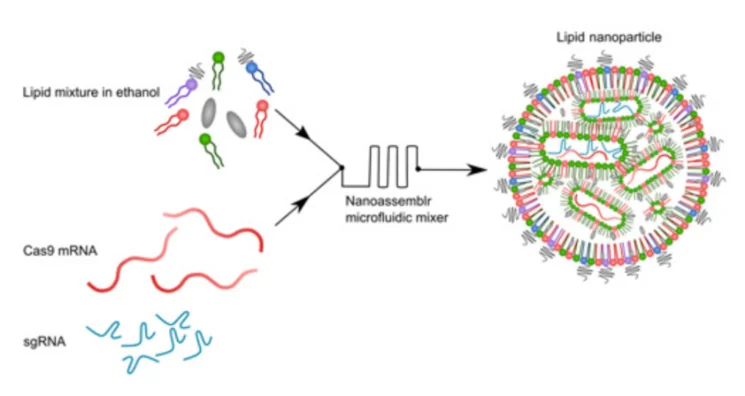
Using a novel library of lipid formulations, the researchers were able to develop a working delivery system. LNPs they had created successfully encapsulated CRISPR-Cas9 systems and delivered them to various cancer cells in vitro, with up to 98% gene editing efficacy. As for the mechanism of action, the researchers aimed for PLK1, a kinase that is essential for mitosis (cellular division). With PLK1 gene cut out, the cells were unable to divide. This resulted in cell cycle arrest and death by apoptosis.
LNPs treat brain and ovarian cancer
Of course, the ultimate test had to be done in vivo. For the first set of experiments, the researchers chose glioblastoma (GBM), a type of aggressive brain cancer. GBM is exceptionally lethal and resistant to treatment, mainly because the blood-brain barrier prevents conventional anti-cancer drugs from reaching the tumor. Only 5% of GBM patients survive for more than five years.
The researchers injected GBM cells into the hippocampi of mice. Following tumorigenesis, CRISPR-carrying LNPs were injected directly into the tumors, overriding the blood-brain barrier. A single injection resulted in 68% gene editing and high rates of apoptosis, while the surrounding healthy tissue remained mostly intact. Tumor growth was significantly reduced following the injection. 30% of mice survived for 60 days, which is when the experiment was terminated, while all the mice in the control group died within 40 days. The researchers claim that this is the highest survival improvement ever achieved in GBM by a single treatment.
Whenever a direct injection into a single non-metastasizing tumor is possible, the non-specificity of LNPs does not pose a problem. However, to engage metastatic cancer, a systemic injection is needed, so the treatment must ensure that CRISPR-carrying LNPs do not kill healthy cells. The scientists managed to coat LNPs with antibodies specific to various cancer cell types, and such LNPs zoom in on their targets while ignoring healthy cells. In a murine model of metastatic ovarian cancer, CRISPR-carrying LNPs were injected into the abdominal cavity (intraperitoneally). Despite being administered systemically, they delivered 82% of gene editing, strong tumor inhibition, and 80% increase in overall survival.
Conclusion
This research constitutes a potential breakthrough in cancer therapy. LNPs demonstrated their ability to deliver DNA-cleaving CRISPR kits directly to cancer cells even when administered systemically. Direct injection of CRISPR-carrying LNPs into brain tumors appears to be a workable way to override the blood-brain barrier and successfully attack treatment-resistant brain cancer. Although more must be done to establish the efficacy and low toxicity of LNP-based delivery systems, the concept has been proven.
Literature
[1] Rosenblum, D., Gutkin, A., Kedmi, R., Ramishetti, S., Veiga, N., Jacobi, A. M., … & Lieberman, J. (2020). CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Science Advances, 6(47), eabc9450.
[2] Hanlon, K. S., Kleinstiver, B. P., Garcia, S. P., Zaborowski, M. P., Volak, A., Spirig, S. E., … & Lööv, C. (2019). High levels of AAV vector integration into CRISPR-induced DNA breaks. Nature communications, 10(1), 1-11.
[3] Ronzitti, G., Gross, D. A., & Mingozzi, F. (2020). Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Frontiers in Immunology, 11.
[4] Puri, A., Loomis, K., Smith, B., Lee, J. H., Yavlovich, A., Heldman, E., & Blumenthal, R. (2009). Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Critical Reviews™ in Therapeutic Drug Carrier Systems, 26(6).